INTRODUCTION
Antibiotics are among the most commonly prescribed drugs to patients of all ages.1 Adverse health and economic outcomes like antibiotic resistance, numerous adverse drug reactions and consequences of both are associated with the use of antibiotics.1,2,3 Drug hypersensitivity reactions that are commonly called “allergy” are the serious problem in the use of antibiotics. These adverse drug reactions occur in response to normal therapeutic doses of a drug and are the result of a specific immunologic response to a medication.4,5,6
Antibiotic allergy has a strong impact on the development of health-care associated infections and antibiotic resistance.7 For instance, inpatients with penicillin allergy have a significantly higher frequency of infections by several agents, including methicillin-resistant Staphylococcus aureus, Enterococcus species and Escherichia coli.8 In patients with a history of penicillin allergy the risk of adverse events triples because they receive alternative antibiotics which often have a greater risk of side effects.7,9 Hospitalizations of patients with a history of penicillin allergy have a longer average length of stay and higher hospital charges in comparing with patients without penicillin allergy in the past.8
Moreover, antibiotic hypersensitivity is a common reason for visits and admissions to hospitals.3,4,10-15 According to different studies, up to 12% of all visits to emergency departments are due to different adverse drug events, including antibiotic allergy.13,14,16,17 In the United States, antibiotics are implicated in 13.7% of all adult emergency department visits. Allergic reactions account for approximately 74% of these visits.3 Anaphylactic shock, angioedema and urticaria which lead to hospitalization in Poland, the most frequently occurs after taking antibiotics.12 In Italy, the main cause of emergency departments visits and hospital admissions caused by drugs was antibiotic hypersensitivity.17 In the United Kingdom, increasing of cases of iatrogenic anaphylaxis which lead to hospitalizations is associated with increased antibiotic prescriptions.18
Receiving medications during hospital stay may contribute to numerous drug-related problems (DRPs) which can result in substantial additional costs, morbidity or mortality.19-23
Little work has been done in this area in Ukraine. The problem of antibiotic allergies considering epidemiological and clinical importance has not been quantified so far. Thus, this is the first study in Ukraine to specify the incidence of hospital admissions caused by antibiotic allergies, describe causative drugs, risk groups, clinical characteristics of patients and evaluate the pharmacotherapy of allergic reactions through the identification of DRPs.
METHODS
Terminology
In this study different terms (adverse drug event, adverse drug reaction and drug-related problem) associated with drug safety and admission to hospitals have been used.
Adverse drug event - any untoward medical occurrence that may present during treatment with a pharmaceutical product but which does not necessarily have a causal relationship with this treatment.24,25
Adverse drug reaction (ADR) can be defined as “an appreciably harmful or unpleasant reaction resulting from an intervention related to the use of a medicinal product”.26
Drug-related problem (DRP) is “an event or circumstance involving drug therapy that actually or potentially interferes with desired health outcomes”.22,25,27
Study design and data collection
This retrospective hospital-based study was carried out in the Unit that provides medical care for adult inpatients with allergy diseases and allergy reactions in one of Lviv City Hospitals (Ukraine). For ethical reasons, we do not specify the name of the hospital and the name of the Unit. Acute drug, insect, food and idiopathic allergic reactions are among the causes for admissions to this Unit.
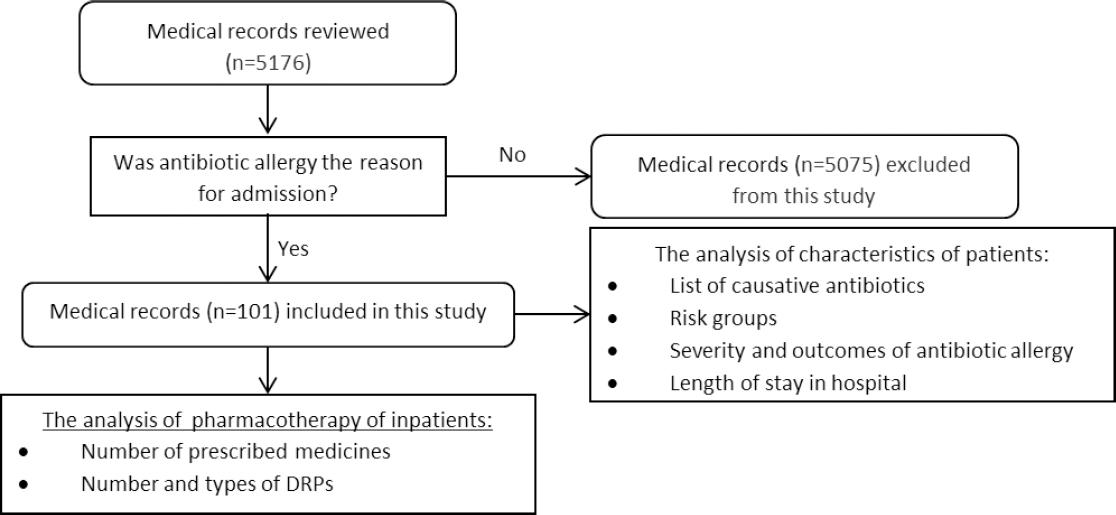
Data were collected by analysis of archival medical records of all patients (n=5176) admitted to this Unit, from January 2015 to December 2017. All medical records were reviewed by research team for the principal diagnosis corresponded to the ICD-10 codes: T88.7 - “Unspecified adverse effect of drug or medicament” and T88.6 - “Anaphylactic shock due to adverse effect of correct drug or medicament properly administered”.28 All hospitalizations with admission diagnosis coded as “T88.7”, “T88.6” and caused by antibiotics were included in this study (Figure 1). The aim of this study did not include determination of causality between antibiotic use and appearance of allergic reaction, laboratory testing and monitoring condition of patients. We did not include visits to hospital which did not result in a hospitalization.
Copies of medical records were acquired through an agreement with the hospital administration. The research team developed a special form (data collection tool) to review and analyse cases of antibiotic allergy. The data collected included medical record number, age and gender, diagnosis and severity, causal antibiotic, medical history, length of stay, comorbidities and health status of patient on admission and discharge. Drugs, dosage and frequency regimen were also entered into developed form.
The evaluation of pharmacotherapy was carried out through the identification of DRPs.27 All medical records included in the study were carefully reviewed by research team. Evaluation of DRPs was done by the Pharmaceutical Care Network Europe (PCNE) DRPs classification v5.01.27 Appropriate doses of drugs, drug indications, contraindications, potential drug-drug interactions (DDIs) were based on drug monographs in the 10th edition of the Ukrainian National Formulary.29 Potential DDIs, drug indications and contraindications were also based on Medscape (“Drug interaction checker”; “Drugs and diseases”).30
Statistical analysis
Descriptive statistics were used to describe demographic and disease characteristics of patients. Percentages and frequencies were calculated for the qualitative variables, while mean, standard deviation (SD) and ranges were expressed for quantitative variables. A 95% confidence interval (CI) was calculated for the proportions. To test the significant difference between groups of patients in 2015, 2016 and 2017 Kruskal-Wallis H tests were used for quantitative variables and Chi-square tests were used for qualitative variables. A value of P less than 0.05 was considered statistically significant. All data analyses were done using Statistica 10 Trial.
RESULTS
A total of 5176 patients (1759, 1864 and 1553 patients in 2015, 2016 and 2017 respectively) were admitted to the Unit during the study period. Antibiotic hypersensitivity reactions were noted as the reason for hospitalization in 101 medical records (37, 36 and 28 patients in 2015, 2016 and 2017 respectively). The incidence of antibiotic allergies was almost 2.0% (95%CI 1.6:2.4) of all admissions to the Unit during study period with tendency towards to not statistically significant decreasing: 2.1% (95%CI 1.5:2.9) in 2015, 1.9% (95%CI 1.3:2.6) in 2016 and 1.8% (95%CI 1.2:2.6) in 2017 (p>0.05).
The admissions included 79 (78.2%; 95%CI 68.9:85.8) females and 22 (21.8%; 95%CI 14.2:31.1) males. The female predominance was established for each year of study period (p>0.05). The mean age of patients was 48.5 years (SD=17.0; range 18-83 years). The length of hospitalization varied from 2 to 13 days, a mean of 8.2 days per patient (SD=2.2). During research period each of our sample used a mean of 5.4 medicines (SD=1.2), from 3 to 12 items. The total number of prescribed drugs was 541. There was no statistical significance in the distribution of all above items in 2015, 2016 and 2017 (p>0.05). The main characteristics of the study population of 2015, 2016 and 2017 are summarized in Table 1.
Table 1. Characteristics of patients
| Characteristics | All patients (n=101) (%) | Distribution of patients by years | |||
|---|---|---|---|---|---|
| 2015 (n=37) (%) | 2016 (n=36) (%) | 2017 (n=28) (%) | p-value | ||
| Gender | 0.2996 | ||||
| male | 22 (21.8) | 11 (29.7) | 7 (19.4) | 4 (14.3) | |
| female | 79 (78.2) | 26 (70.3) | 29 (80.6) | 24 (85.7) | |
| Age in years | 0.9415 | ||||
| mean (SD) | 48.5 (SD=17.0) | 48.1 (SD=16.2) | 47.9 (SD=15.9) | 49.7 (SD=19.7) | |
| min-max | 18-83 years | 18-76 years | 23-81 years | 20-83 years | |
| less than 20 | 1 (1.0) | 1 (2.7) | 0 | 0 | |
| 20-29 | 16 (15.8) | 5 (13.5) | 4 (11.1) | 7 (25.0) | |
| 30-39 | 18 (17.8) | 5 (13.5) | 9 (25.0) | 4 (14.3) | |
| 40-49 | 17 (16.8) | 8 (21.6) | 7 (19.4) | 2 (7.1) | |
| 50-59 | 22 (21.8) | 10 (27.0) | 8 (22.2) | 4 (14.3) | |
| 60-69 | 11 (10.9) | 2 (5.4) | 2 (5.6) | 7 (25.0) | |
| more than 69 | 16 (15.8) | 6 (16.2) | 6 (16.7) | 4 (14.3) | |
| Length of hospitalization, days | 0.1569 | ||||
| mean (SD) | 8.2 (SD=2.2) | 8.4 (SD=2.5) | 7.6 (SD=2.2) | 8.5 (SD=1.8) | |
| min-max | 2-13 days | 2-12 days | 2-11 days | 6-13 days | |
| 1-3 | 4 (4.0) | 2 (5.4) | 2 (5.6) | 0 | |
| 4-6 | 15 (14.8) | 6 (16.2) | 7 (19.4) | 2 (7.1) | |
| 7-9 | 54 (53.5) | 16 (43.2) | 20 (55.6) | 18 (64.3) | |
| 10-12 | 26 (25.7) | 13 (35.1) | 7 (19.4) | 6 (21.4) | |
| more than 12 | 2 (2.0) | 0 | 0 | 2 (7.1) | |
| Number of prescribed medications | 0.4125 | ||||
| mean (SD) | 5.4 (SD=1.2) | 5.3 (SD=1.8) | 5.2 (SD=1.4) | 5.6 (SD=1.3) | |
| min-max | 3-12 items | 3-12 items | 3-9 items | 4-9 items | |
| Health status on admission* | |||||
| moderate-to-severe | 98 (97.0) | 36 (97.3) | 35 (97.2) | 27 (96.4) | |
| severe | 3 (3.0) | 1 (2.7) | 1 (2.8) | 1 (3.6) | |
| Health status on discharge | 0.2835 | ||||
| healthy | 70 (69.3) | 29 (78.4) | 24 (66.7) | 17 (60.7) | |
| improving | 31 (30.7) | 8 (21.6) | 12 (33.3) | 11 (39.3) | |
| Manifestation of allergic reaction | 0.0930 | ||||
| urticaria | 37 (36.6) | 14 (37.8) | 13 (36.1) | 10 (35.7) | |
| angioedema | 11(10.9) | 2 (5.4) | 8 (22.2) | 1 (3.6) | |
| urticaria w/angioedema | 53 (52.5) | 21 (56.8) | 15 (41.7) | 17 (60.7) | |
| Co-morbidities | 0.7419 | ||||
| yes | 31 (30.7) | 12 (32.4) | 12 (33.3) | 7 (25.0) | |
| no | 70 (69.3) | 25 (67.6) | 24 (66.7) | 21 (75.0) | |
| Previous drug allergy | 0.1610 | ||||
| yes | 36 (35.6) | 12 (32.4) | 17 (47.2) | 7 (25.0) | |
| no | 65 (64.4) | 25 (67.6) | 19 (52.8) | 21 (75.0) | |
SD standard deviation; urticaria w/angioedema urticaria with angioedema;
*p-value was not determined
In 49 (48.5%; 95%CI 38.5:58.7) medical records beta lactams (in particular penicillins and cephalosporins) were noted as the cause of allergic reaction, in 14 (13.9%; 95%CI 7.8:22.2) - fluoroquinolones, in 8 (7.9%; 95%CI 3.5:15.0) - macrolides, in 4 (3.9%; 95%CI 1.1:9.8) - chloramphenicol, in 3 (2.9%; 95%CI 0.6:8.4) - sulfonamides, in 2 (1.9%: 95%CI 0.2:7.0) - lincosamides. Tetracyclines (doxycycline), nitrofuran derivatives (furazidine), 8-hydroxyquinoline derivatives (nitroxoline) and pyridopyrimidines (pipemidic acid) were noted as causative drug only once. In 17 (16.8%; 95%CI 10.1:25.6) medical records specific names of causative antibiotics were missed. Overall comparison of antibiotics that caused allergy related hospitalizations in 2015, 2016 and 2017 is shown in Table 2.
Table 2. Antibiotics that led to allergic reactions and hospitalization in 2015, 2016 and 2017
| Antibiotics | All cases (n=101) | Distribution of cases by years n(%) | ||
|---|---|---|---|---|
| 2015 (n=37) | 2016 (n=36) | 2017 (n=28) | ||
| Penicillins | 34 (33.7) | 15 (40.5) | 9 (25.0) | 10 (35.7) |
| Cephalosporins | 15 (14.8) | 4 (10.8) | 3 (8.3) | 8 (28.6) |
| Fluoroquinolones | 14 (13.9) | 3 (8.1) | 7 (19.4) | 4 (14.3) |
| Macrolides | 8 (7.9) | 3 (8.1) | 3 (8.3) | 2 (7.1) |
| Chloramphenicol | 4 (3.9) | 2 (5.4) | 2 (5.6) | 0 |
| Sulfonamides | 3 (2.9) | 0 | 3 (8.3) | 0 |
| Lincosamides | 2 (1.9) | 1 (2.7) | 0 | 1 (3.6) |
| Tetracyclines | 1 (1.0) | 0 | 1 (2.8) | 0 |
| Pyridopyrimidines | 1 (1.0) | 0 | 1 (2.8) | 0 |
| 8-hydroxyquinoline derivatives | 1 (1.0) | 0 | 1 (2.8) | 0 |
| Nitrofuran derivatives | 1 (1.0) | 1 (2.7) | 0 | 0 |
| Antibiotics (names missed) | 17 (16.8) | 8 (21.6) | 6 (16.7) | 3 (10.7) |
Antibiotic allergic reactions in 37 (36.6%; 95%CI 27.8:46.8) cases were manifested as urticaria, 11 (10.9%; 95%CI 5.6:18.7) - as angioedema and 53 (52.5%; 95%CI 42.3:62.5) - as urticaria with angioedema. Moderate to severe health status of patients on admission were noted in 97% (95%CI 91.6:99.4) of the medical records. About 36% (95%CI 26.4:45.8) of patients had allergic reactions in the past to different drugs, but names of drugs were noted only in 44.4% of medical records.
30.7% (95%CI 21.9:40.1) of patients had from 1 to 5 concomitant diseases, such as hypertension (n=15), gastroesophageal reflux disease (n=4), gastritis (n=4), chronic pyelonephritis (n=3), diabetes mellitus (n=3), chronic pancreatitis (n=3), urinary stone disease (n=3), community-acquired pneumonia (n=3), gallstone disease (n=2), chronic obstructive pulmonary disease (n=2), chronic prostatitis (n=2) and congestive heart disease (n=2). Acute cystitis, chronic glomerulonephritis, chronic tonsillitis, acute bronchitis, chronic venous insufficiency of the lower extremities and peptic ulcer disease were recorded as co-morbidity only once.
The total number of identified DRPs was 400, a mean of 4.0 DRPs per patient (SD=1.8). All patients had at least one DRP. Out of these 400 DRPs, 100 (25.0%; 95%CI 20.8:29.5) were classified as inappropriate route of drug administration, 94 (23.5%; 95%CI 19.4:28.0) - prescription of several drugs of the same therapeutic group, 76 (19.0%; 95%CI 15.3:23.2) - insufficient frequency of drug daily administration and, consequently, insufficient daily dosage, 64 (16.0%; 95%CI 12.6:20.0) - potential DDIs, 64 (16.0%; 95%CI 12.6:20.0) - inappropriate drug prescription, 1 (0.25%; 95%CI 0.01:1.38) - insufficient single drug dosage, 1 (0.3%; 95%CI 0.01:1.38) - contraindicated use of medicine. There was no significant difference of total number of identified DRPs in 2015 (n=143 DRPs), 2016 (n=132 DRPs) and 2017 (n=125 DRPs) (p>0.05). Table 3 summaries 7 items of identified DRPs.
Table 3. Identified drug-related probelms in 2015, 2016 and 2017
| Items of DRPs | Distribution of DRPs by years; n (95%CI) | |||||
|---|---|---|---|---|---|---|
| 2015 (n=143) | 2016 (n=132) | 2017 (n=125) | ||||
| Inappropriate route of drug administration (n=100) | 37 | 25.9 (18.9:33.9) | 35 | 26.5 (19.2:34.9) | 28 | 22.4 (15.4:30.7) |
| Duplication of therapeutic group (n=94) | 33 | 23.1 (16.5:30.9) | 32 | 24.2 (17.2:32.5) | 29 | 23.2 (16.1:31.6) |
| Insufficient frequency of drug administration or insufficient daily dosage (n=76) | 22 | 15.4 (9.9:22.3) | 26 | 19.7 (13.3:27.5) | 28 | 22.4 (15.4:30.7) |
| Potential DDIs (n=64) | 26 | 18.2 (12.2:25.5) | 17 | 12.9 (7.7:19.8) | 21 | 16.8 (10.7:24.5) |
| Inappropriate drug (n=64) | 24 | 16.8 (11.1:23.9) | 21 | 15.9 (10.1:23.3) | 19 | 15.2 (9.4:22.7) |
| *Insufficient single drug dosage (n=1) | 0 | 0 | 1 | 0.8 (95%CI 0.0:4.2) | 0 | 0 |
| *Contraindicated drug usage (n=1) | 1 | 0.6 (95%CI 0.0:3.8) | 0 | 0 | 0 | 0 |
SD standard deviation; DDIs drug-drug interactions; DRPs Drug-related problems; CI confidence interval;
*P-level was not determined
As a result of pharmacotherapy, 70.3% (95%CI 60.4:79.0) of patients were healthy on discharge. The health status of remaining patients (29.7%; 95%CI 21.0:39.6) was improved.
DISCUSSION
This was the first study in Ukraine to specify the incidence, causative drugs, risk groups, clinical burden and antibiotic allergy related admissions to the hospital and DRPs which occurred during the hospitalization stay.
According to results of this study, antibiotic allergy associated admission rates, risks groups of patients (age, gender, comorbidities), manifestation of antibiotic allergy, length of hospitalization, number of prescribed drugs and types of DRPs have not changed substantially during the study period. Thus, a permanent problem of antibiotic allergy exists in Ukraine that might be solved (at least in part) by effective strategies to avoid unnecessary prescribing of antibiotics, minimize the burden of antibiotic allergy related admissions and improve pharmacotherapy of patients with antibiotic allergy manifestations because, according to some researches, up to 30% of all adverse drug related admissions are potentially avoidable.14
In England adverse drug reactions related admissions, including drug allergy, have represented 0.9% of total hospital admissions.11 In Croatia the drug hypersensitivity incidence has been detected 0.87% of all emergency department visits. Beta-lactams were identified as the leading cause of drug hypersensitivity.4
In the Netherlands about 5% of all acute hospitalizations are definitely or probably related to adverse drug reactions, including antibiotic hypersensitivity.14 In this study, we managed to calculate the incidence (about 2%) of antibiotic allergy related hospitalizations among all admissions to one Unit. Some of them may be due to self-medication of antibiotics, opportunity of dispensing without prescription and an increase in inappropriate antibiotics use.
The frequency of antibiotic allergies that led to hospitalizations was higher in women. Similarly, female predominance among patients with antibiotic allergy has been established in numerous previous studies.3-5,7,17,31-34
In this study, the majority of patients were in the 50-59 age bracket. Slightly different results have been obtained in the United Kingdom and the United States where the highest rates of admissions were in groups aged 60 years and 65 years respectively.10,18 In some studies, emergency department visits caused by antibiotic allergy were more frequent among younger patients. However, these patients had symptoms that regressed following emergency department treatment and did not require subsequent hospitalization.3,4
Almost all patients (97%) presented with moderate-to-severe allergic reactions. The most common clinical manifestations were urticaria with angioedema (labial, larynx or face edema). Our findings are very similar to what has been previously reported in Croatia and differ from data in the United States, Italy, Greece and some other countries where mild allergic reactions (like rash or pruritus) have been the most common symptoms of antibiotic allergy related emergency department visits.3,4,15-17
Hypertension, gastroesophageal reflux disease and gastritis were the most frequent co-morbidities in this study. Contrary to these results, in other studies viral diseases, rhinitis, asthma and chronic urticaria have been defined as co-morbidities in patients with an increased risk for drug allergy.33,35,36
Approximately a third of patients in this study had had an allergy to different drugs in the past but the specific drug names were often not documented in the medical records. Incomplete medical records are a serious problem in Ukraine. Previous reactions to drugs belong to patient-related factors associated with an increased risk of drug allergy.36
In 49 (48.5%) cases beta lactams allergic reactions were documented as a reason for hospitalization. Of these, 34 antibiotics were penicillins (33.7%) and 15 cephalosporins (14.9%). Thus, penicillin derivatives were most frequently associated with hospitalization caused by allergic reactions. This was expected when compared with other studies and may be due to the higher prescription rate of these drugs.4,12,32,36,37
As described in scientific literature, the duration of 43% adverse drug reactions, including antibiotics allergic reactions, has lasted more than 2 days.33 In present study more than 53% of patients were admitted to hospital from 7 to 9 days. The treatment of inpatients included withdrawal of causative antibiotic and pharmacotherapy of antibiotic allergy and co-morbidities if necessary. Antihistamines and corticosteroids were administrated to all inpatients according to Ukrainian guidelines for the management of drug allergy.38 Each patient took at mean 5.4 medicines (SD=1.2), from 3 to 12 items. In patients with four or more drugs prescribed during their hospital stay the risk of developing adverse drug reactions is in three times higher compared to patients receiving from one to three drugs.39,40 Concomitant prescribing of several drugs is also often associated with numerous DRPs.19
The next step of this study was to evaluate the treatment of antibiotic allergy through the identification of DRPs. The systemic review by Nivya et al. states that antibiotics have a high profile of DRPs. At the same time, there is insufficient data about DRPs associated with pharmacotherapy of antibiotic allergy in literature.22
Totally 400 DRPs of 7 items were identified in this study. DRPs of item “Inappropriate route of drug administration” were the most frequent with a percentage of 25.0%. Clemastine (n=92) and hydrocortisone acetate suspension for injection (n=8) were involved in this type of DRPs. The solution in the ampoule of clemastine may be diluted 1:5 with isotonic saline or 5 % glucose and must be given slowly (over 2-3 minutes) by intravenous injections.29,30 However, in this study clemastine was given by intravenous drip rather than a jet injection because isotonic saline was used in much bigger amounts. Hydrocortisone acetate suspension for injection is indicated for the local treatment by intra-articular or periarticular injections.29,30 But in this study it was given by intramuscular injection for patients without any joint diseases. These DRPs may change the efficacy of pharmacotherapy and lead to different adverse events.41
In the present study, 23.5% of total DRPs were identified as duplication of therapeutic group due to prescriptions of 2 or 3 histamine H1 receptor antagonists (n=85) at the same time or co-administration of 2 corticosteroids (n=9). The concomitant use of these medicines may result in serious adverse effects.29,30 Thus, monitoring of this practice is required. Besides, prevention of duplicate prescriptions will contribute to cost saving among hospitalized patients.21
The third subset of DRPs included insufficient frequency of drug administration and insufficient therapeutic effect as a consequence. Clemastine (n=76) was implicated in insufficient frequency of administration. This medicine was prescribed only once a day (total daily dose 2 ml) rather than twice a day (total daily dose 4 mg).29,30 It might result in ineffective treatment due to subtherapeutic concentration.21
The total number of identified potential DDIs was 64. In this retrospective study the term “potential DDIs” was used because the judgment about DDIs was based only on theoretical consideration due to absence of patients' condition monitoring. This term refers to the theoretical possibility that one drug can change the intensity of the pharmacological effect of another drug.42 Most of detected potential DDIs (53.1%) were between glucocorticoids (dexamethasone/hydrocortisone) and diuretics (furosemide/hydrochlorothiazide). Concomitant use of these drugs may result in hypokalaemia by pharmacodynamic synergism.29,30 Potential pharmaceutical DDIs between (1) dexamethasone and theophylline, (2) metamizole with diphenhydramine were also identified. These DDIs are still common in Ukraine because the medicines are often mixed in one syringe or drug container. Different previous studies consider DDIs as serious problem which might increase the possibility of the adverse drug reactions occurrence.21,22,39,40,43
Prescriptions of inappropriate drugs were related to levocetirizine (n=50), fexofenadine (n=6) and hydrocortisone acetate suspension for injection (n=8). In fact, both of histamine H1 receptor antagonists are indicated for allergic rhinitis and chronic urticaria, not for acute allergic reaction.29,30 Prescriptions of inappropriate drugs often cause adverse drug reactions especially in the elderly.43,44
One DRP of item “Insufficient single drug dose” was detected due to clemastine. Additionally, one case of contraindicated medication use was identified because ceftriaxone was administrated to inpatient with anaphylactic reaction to ampicillin. Thus, administration of ceftriaxone was contraindicated due to the risk of cross-allergy.29
The high incidence of different DRPs has shown that further education of healthcare workers are required in this field.
Also implementation of clinical decision support system in hospitals and improvement of communication between the healthcare team members are needed in Ukraine. All above make the pharmacotherapy of antibiotic allergy an important area requiring further investigations.
Limitations
The methodological limitations of this study include its retrospective design. Information about causality between antibiotic use and appearance of allergic reaction was based on data in arhival medical records. Some data were missing in medical records, particularly the reasons for antibiotics usage before allergic reactions appearance and specific names of antibiotics.
The real number of DRPs is most likely more than we identified. DDIs can be considered only as potential because we did not monitor the condition of patients. Some DRPs can be attributed to another item of DRPs or to several items at the same time. Thus, there is a subjective factor.
Another notable limitation is that only the medical records from one hospital were considered. Therefore, the findings cannot be statistically generalized and further investigations are required.
CONCLUSIONS
Antibiotic allergy is a common reason for admissions to the hospital. The causative drugs and risk groups of antibiotic allergy related hospitalizations were defined. The treatment of antibiotic allergy is associated with numerous DRPs which can lead to new adverse events, increase the duration of treatment and incur additional costs. Our results could be useful for development and/or improvement of the risk management strategies for antibiotic administration and antibiotic allergy treatment.