INTRODUCTION
Antibiotics are among the most prescribed drugs worldwide and their consumption is continuously increasing.1,2 Antibiotics misuse is contributing to the growing problem of antibiotic resistance now considered a serious threat to public health.3,4 It is estimated that 700,000 people die each year from drug-resistant bacteria globally. The WHO projected 10 million deaths in 2050 if the problem is not addressed by all stakeholders.4 The WHO suggested a multi-level strategy addressed to governments, to health professionals as well as to veterinarians and the general public.5
In Lebanon, national data on resistance patterns and continuity of surveillance are lacking.6 However, a retrospective study of the susceptibility tests in Lebanon between 2011 and 2013 has established a significant increase of antimicrobial resistance with (MRSA) prevalence rate at 27.6%, penicillin-resistant S. pneumoniae at 53.8% and extended-spectrum beta-lactamase (ESBL) producing Escherichia coli (E. Coli) at 32.5%.7 Susceptibility profiles reported in a university medical center in 2017 showed similar results where the figures were 30% for MRSA, 48% for Penicillin-resistant S. pneumoniae and 29.5% for ESBL.8
As for antibiotics misuse and overuse, it is proved at different levels of the continuum of care starting at the community level and pharmacies as well as in hospital settings.9-11 Thus, improving the use of antibiotics is an important patient safety and public health issue as well as a national priority.
One of the evidence-based strategic interventions to reduce the misuse of antibiotics is the implementation of hospital-based programs dedicated to improving antibiotic use, commonly referred to as, antibiotic stewardship programs (ASPs). These programs are demonstrated to both optimize the treatment of infections and reduce adverse events associated with antibiotic use. They also help clinicians improve the quality of patient care as well as patient safety.12
Multiple templates for ASP were adopted across hospitals to optimize antibiotic prescribing. The Lebanese Ministry of Public Health (MoPH) enforced the implementation of evidence-based practices in hospitals to limit antibiotics misuse and overuse through its hospital's accreditation program and recommendations for implementing stewardship programs were made.13 Despite the fact that the policy does not directly target individual physicians, the MoPH accreditation system addresses both private and public hospitals. Many hospitals have started implementing ASPs according to their available resources to improve their accreditation level.14,15 In a study conducted in 2014 addressed to 158 Lebanese hospitals, 65% of the 58 participating hospitals seemed to have an antimicrobial management program, with most adopting a restrictive approach rather a persuasive approach.15 Designing an effective hospital-based program must take into consideration several factors like the size and types of care provided. It also needs to adapt the interventions to the Lebanese context, taking into account the complexity of the medical decision-making surrounding antibiotic use, and the acceptability of physicians. Thus, implementation flexibility is required for these programs.1
In order to find the best way to implement ASP in Lebanon, our objectives were to describe implemented ASPs, and to examine physicians' attitudes towards these programs as well as reported barriers to initiating or sustaining an ASP.
METHODS
Survey design and procedure
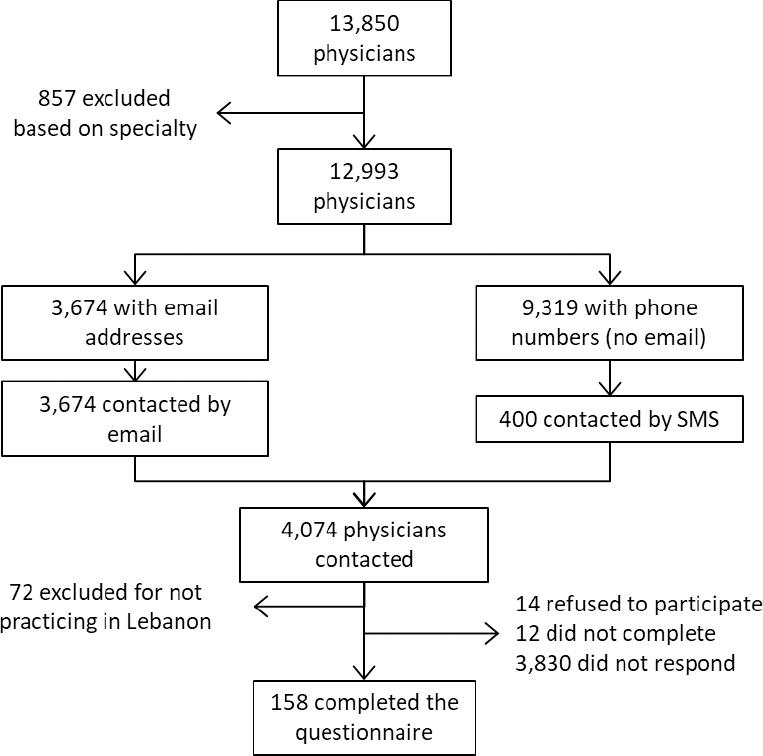
A cross-sectional pilot study was carried out between July and November 2018. Google Forms was used to conduct the web-based survey. The list of physicians was obtained from the two orders of physicians in Lebanon (Beirut and Tripoli) with a total population of 13850 physicians. Exclusion criteria were physicians not practicing in Lebanese hospitals and physicians with the following specialties (as they do not prescribe antibiotics): Anatomical and Clinical Pathology, Laboratory Medicine, Legal Medicine, Diagnostic Radiology, Nuclear Medicine, Physical Medicine and Rehabilitation, Psychiatry, Sport Medicine.
After excluding the physicians based on their specialties, only physicians with email addresses provided in the orders' lists were retained. As it was impossible to discern the physicians who work in hospitals, and since their response rates in health services research are usually low, no sampling was done initially, as we sent an email with the link to the online survey to the entire eligible population who had email addresses registered in the order.16-18 This was done in order to have the highest number possible of completed questionnaires. Reminder emails were sent every two weeks for 7 weeks. Also, to gain more responses, the survey was sent by text messages (SMS) to 400 physicians randomly selected among eligible physicians with mobile numbers. The random sample was generated in SPSS. Two reminder messages were also sent after one week and after two weeks.
Questionnaire/survey tool
A review of successful ASPs in acute care hospitals was conducted by the CDC in 2014. The core elements of these ASPs were identified with an updated revision in 2019. These elements include: leadership commitment (a single program leader responsible for outcomes), a pharmacy leader, specific interventions to improve prescribing (such as: antibiotic "time outs, preauthorization, prospective audit and feed-back, automatic stop orders, as well as disease specific interventions), tracking antibiotic use and resistance, reporting data back to providers, and education.12
The survey tool (Online appendix) was adapted from a questionnaire that was used to assess Canadian intensivists knowledge, attitudes and practices (KAP) with ASP in 2012.19
Since our target population consisted of physicians, the questionnaire was shared in English, as it constitutes the most widely used medical language among them. The following definition of ASP was provided at the beginning of the questionnaire: "Antimicrobial stewardship program (or Antimicrobial control program): is a multifaceted approach for preventing the emergence of antimicrobial resistance through the appropriate selection, dosing, route and duration of antimicrobial therapy".20
The questionnaire had seven sections. The first addressed physician and hospital characteristics (such as physician's age, specialty, and years of experience as well as hospital size and accreditation status…). Participants were asked the following question: "Are you familiar with the concept of an Antimicrobial Stewardship Program (ASP)".
This was followed by the following sections:
Practices
Participants were asked to choose which of the nine ASP interventions were implemented in their hospital. The number of ASP components was noted (with a minimum of 0 and a maximum of 9 components). These interventions were selected based on the CDC core-elements of hospital ASPs and the above-mentioned survey tool.12 Additional interventions were added based on other studies.21-23
Attitude
Participants' attitude was evaluated using seven items. These items were selected by adapting the items used in the above-mentioned survey tool and combined with pertinent questions that were found in other articles assessing the physicians' attitude towards ASP and adapted to the Lebanese context.21-23 In fact, when asking about time spent interacting with ASP members, the original questionnaire had different statements for each member type, while we merged all members into one statement.19 We also added the following statements based on the experts' recommendations: "I feel that gaining approval for restricted ATB makes the team think more carefully about ATB choice", "I feel that the treating physician is in the best position to know the best ATB", and "I feel that ATB guidelines and ATB committee are an obstacle more than a help to clinical care".
Likert scale statements were used. For positive statements such as "Gaining approval for restricted ATB makes the team think more carefully about ATB choice", 0 indicated strongly disagree, 1 disagree, 2 neutral, 3 agree and 4 strongly agree. For the negative statements such as: "I feel that an ASP affects/would affect my autonomy in a negative way", the codes were inverted with 4 indicating strongly disagree, 3 disagree, 2 neutral, 1 agree and 0 strongly agree. This was done to ensure that a higher average be associated with more positive attitudes towards ASP. An average score was also calculated for each statement. A Cronbach alpha was calculated for the attitudes questionnaire, and was of 0.651.
We also assessed respondents' preferences for a computer approval program and a faxed/written one for restricted antibiotics.
Interaction method usefulness: Usefulness of ASP interventions was also evaluated using a 4-point Likert-scale items (0 = Not useful, 1 = Neutral, 2 = Somewhat useful, 3 = Very useful) with a non-applicable option in case the intervention was not applied in the respondent's hospital. An average score was also calculated for each statement.
Importance of decision tools: Respondents' were asked about the importance of decision tools for antibiotic prescribing integrated to the ASPs.
Recent change in prescribing habits
Participants were asked if they noticed a change in their prescribing habits. Of those who did, respondents were invited to identify to describe the change, and specify the reason behind it.
Barriers
One section was included regarding the perceived barriers to ASP implementation where multiple answers were possible.
Finally, an open-ended question was added to collect participants' comments on ASP. Content and face validity were checked by two experts in the field: an ID physician and a lab director.
A pilot study was done on 5 physicians to verify that questions were comprehensible and relevant to the Lebanese context. Subsequent changes were made accordingly. These 5 questionnaires were disregarded. A completion time of 10 minutes was also estimated during the test period.
Ethical considerations
Respondents were informed at the beginning of the questionnaire that the survey was completely voluntary and anonymous. Confidentiality of the responses was guaranteed to the participants. Only participants who chose "Yes" to the question "Do you consent to participate in the study" had access to the questionnaire. The study being observational and respecting participants' anonymity and confidentiality, the Internal Review Board (IRB) of the Lebanese university waived the need for an official approval.
RESULTS
A total of 256 responded to the survey. Of those, 14 disagreed to participate and 72 were not practicing in a Lebanese hospital. A total of 158 questionnaires were filled. A response rate of 4% was obtained, with a completion rate of 86% (158 from the 184 that clicked on the link) (Figure 1).
The majority of respondents had a non-surgical specialty (n=98, 63%). Most of them were not members of an ASP team (n=139, 88%) and more than half of the respondents (n=85, 53.8%) worked in university private hospitals. The majority of physicians who were ASP members were not infectious disease specialists (n=15, 78.9%). Two-thirds of the respondents (n=104, 65.8%) said they were familiar with the ASP concept, while the others had never heard about the concept (Table 1).
Table 1. Characteristics of the sample
| Physicians Characteristics | n (%) |
|---|---|
| Age | |
| ≤ 35 years | 57 (36.1) |
| 36-50 years | 70 (44.3) |
| 51-64 years | 25 (15.8) |
| ≥ 65 years | 6 (3.8) |
| Experience | |
| < 2 years | 15 (9.5) |
| 2-5 years | 49 (31.0) |
| 6-10 years | 26 (16.5) |
| 11-20 years | 38 (24.1) |
| > 20 years | 30 (19.0) |
| Specialty | |
| Non-surgical | 98 (62.8) |
| Surgical | 50 (32.1) |
| Infectious Disease | 8 (5.1) |
| ASP Membership | |
| No | 139 (88.0) |
| Yes | 19 (12.0) |
| Member of ASP (n=19) | |
| Infectious disease specialist | 4 (21.1) |
| Other | 15 (78.9) |
| Are you familiar with the concept of ASP? | |
| Yes | 104 (65.8) |
| Hospitals Characteristics | |
| Type | |
| University Private Hospital | 85 (53.8) |
| Private Hospital | 61 (38.61) |
| University Public Hospital | 6 (3.8) |
| Public Hospital | 6 (3.8) |
| Size | |
| < 100 beds | 37 (23.4) |
| 100-199 beds | 73 (46.2) |
| ≥ 200 beds | 48 (30.4) |
| Accreditation status | |
| Not Accredited | 13 (8.2) |
| Accredited | 145 (91.8) |
Participants responded that their place of work had on average 5.79 (SD=2.98) implemented ASP components in place. Almost all respondents (n=146, 92.4%) confirmed their place of work had implemented at least one component of ASP while 27.8% alleged all nine components were currently in place (n=44). The most frequently implemented practice was having an ID physician or ID pharmacist rounding (n=130, 82.3%), followed by audit and feedback for some prescribed antibiotics (n =119, 75.3%), restricting antimicrobials to ID consultants (n=113, 71.5%), and surgical prophylaxis interventions (n=112, 70.9%).
More than half of the respondents (55%) who answered about ASP duration (n=49) were unsure of the duration of the ASP implementation, while 27% (n=13) answered that ASP was implemented since more than four years.
Interaction frequency with the ASP team was once or twice per week (n=42, 86%) in most of the responses. Only 41% of the respondents (n=65) reported that they had regular educational programs on the appropriate use of antimicrobials for hospital staff. Local antibiograms to assess local resistance and susceptibility patterns were developed and monitored by hospitals laboratory in 71% of the responses (n=112). Of those who responded about the team composition (n=49), 96% had an ID physician in their ASP team, while 63% had an ASP pharmacist, and 65% an infection control officer (Table 2).
Table 2. Antimicrobial stewardship program implementation
| Antimicrobial stewardship program component (n=158) | n (%) |
|---|---|
| ID physician / pharmacist rounding | 130 (82.3) |
| Audit and feedback for some ATBs prescribed | 119 (75.3) |
| Antimicrobials restricted to ID consultants | 113 (71.5) |
| Specific intervention for Surgical prophylaxis | 112 (70.9) |
| Specific intervention for Urinary tract infections | 93 (58.9) |
| Specific interventions for intraabdominal infections | 91(57.6) |
| Time-sensitive Automatic Stop Order | 89 (56.3) |
| Specific intervention for community acquired pneumonia | 86 (54.4) |
| Specific interventions for skin and soft tissue infections | 82 (51.9) |
| ASP duration (n =49 ) | |
| < 1 year | 5 (10.2) |
| 1 to 2 years | 3 (6.1) |
| 3 to 4 years | 1(2.0) |
| > 4 years | 13 (26.5) |
| Unsure | 27 (55.1 |
| Interaction frequency (n = 49) | |
| Once or twice per week | 42 (85.7) |
| 3 to 4 times per week | 5 (10.2) |
| > 4 times per week | 2 (4.1) |
| Regular education programs (n=158) | |
| Yes | 65 (41.1) |
| No | 93 (58.9) |
| Local antibiograms developed by hospital's lab (n=158) | |
| Yes | 112 (70.9) |
| No | 46 (29.1) |
| Team composition (n=49) | |
| Infectious disease physician | 47 (95.9) |
| ASP pharmacist | 31 (63.3) |
| Infection control officer | 32 (65.3) |
| Other | 7 (16.3) |
Agreement to attitude statements are presented in Table 3. Almost all respondents agreed that asking for approval for restricted antibiotic (ATB) made the team think more carefully about drug choice (n=151, 95.6%). They also agreed that ASP was beneficial to their patients (n=149, 94.3%), and that ASP increased their knowledge of appropriate antimicrobial use (n=144, 91.1%). Importantly, one third considered that ASP affected their autonomy in a negative way (n=53, 33.7%), more than half (n=85, 53.8%) believed that the physician was in the best position to choose the right antibiotic for his patient, while 23.4% of the participants (n=37) agreed that ATB guidelines and committee were an obstacle to clinical care.
Table 3. Percentage of agreement to attitude statements (n=158)
| Attitude statement | Strongly disagree | Disagree | Neutral | Agree | Strongly agree | Average |
|---|---|---|---|---|---|---|
| I feel that gaining approval for restricted ATB makes the team think more carefully about ATB choice | 1 (0.6) | 1 (0.6) | 5 (3.2) | 67 (42.4) | 84 (53.2) | 3.5 |
| I feel that my patients benefit/would benefit having an antimicrobial stewardship program in place | 1 (0.6) | 1 (0.6) | 7 (4.4) | 54 (34.2) | 95 (60.1) | 3.5 |
| I feel that an antimicrobial stewardship program increases/would increase my knowledge of appropriate antimicrobial use | 1 (0.6) | 3 (1.9) | 10 (6.3) | 64 (40.5) | 80 (50.6) | 3.4 |
| I feel that time spent interacting with the antimicrobial stewardship program physician /pharmacist is/would be an efficient use of my time | 1 (0.6) | 6 (3.8) | 10 (6.3) | 69 (43.7) | 72 (45.6) | 3.3 |
| I feel that the treating physician is in the best position to know the best ATB | 2 (1.3) | 26 (16.5) | 45 (28.5) | 46 (29.1) | 39 (24.7) | 1.4 |
| I feel that an antimicrobial stewardship program affects/would affect my autonomy in a negative way | 15 (9.5) | 43 (27.2) | 47 (29.7) | 29 (18.4) | 24 (15.2) | 2.0 |
| I feel that ATB guidelines and ATB committee are an obstacle more than a help to clinical care | 29 (18.4) | 51 (32.3) | 41 (25.9) | 21 (13.3) | 16 (10.1) | 2.4 |
A computer approval program for restricted antibiotics was preferred with 55.7% (n=88) stating a positive attitude towards it, while 44.3% (n=70) of the respondents had positive attitudes towards a faxed or written one.
Antibiotic rounds with ASP physician/pharmacist, standard meeting time on rounds and prospective audit and feedback were rated as the most useful elements (Table 4).
Table 4. Interaction method usefulness
| Intervention | Not useful | Neutral | Somewhat useful | Very useful | Average |
|---|---|---|---|---|---|
| Antibiotic rounds with antimicrobial stewardship program physician/pharmacist (n=154) | 7 (4.5) | 6 (3.9) | 40 (26.0) | 101 (65.6) | 2.5 |
| Standard meeting time on rounds (n=154) | 3 (1.9) | 13 (8.4) | 50 (32.5) | 88 (57.1) | 2.4 |
| Prospective audit and feedback (n=154) | 5 (3.2) | 9 (5.8) | 54 (35.1) | 86 (55.8) | 2.4 |
| Written feedback in progress notes on patient chart (n=155) | 7 (4.5) | 15 (9.7) | 52 (33.5) | 81 (52.3) | 2.3 |
| Written suggestions in doctors' orders (n=155) | 4 (2.6) | 6 (3.9) | 65 (41.9) | 80 (51.6) | 2.4 |
| Verbal feedback (outside of formal rounds) (n=152) | 3 (2) | 13 (8.6) | 74 (48.7) | 62 (40.8) | 2.3 |
Almost all respondents highlighted the importance of having rapid microbiological tests (n=156, 98.7%) and local resistance data in their treatment decision (n=155, 98.1%) (Table 5).
Table 5. Importance of decision tools (n=158)
| Decision tool | n (%) |
|---|---|
| Rapid microbiological diagnostic tests is important for ATB treatment decision | 156 (98.7) |
| Local resistance data is an important information for optimal ATB use | 155 (98.1) |
| National resistance data is an important information for optimal ATB use | 147 (93.0) |
| Prefer more guidance from ID experts on ATB prescribing | 139 (88.0) |
| Local guidelines development more useful than the international ones | 136 (86.1) |
About 75% of respondents think that their antimicrobial use has changed during the last two years (n=118, 74.7%). Among physicians who perceived a change, the most reported prescribing change was the use of more targeted therapy (n=78, 66.1%) and shorter treatment durations (n=70, 59.32%). It is noteworthy that 96.6% of participants (n=114) reported that their higher awareness regarding ATB resistance influenced the change in their prescribing pattern. Other reasons for these changes were receiving ID consults (n =103, 87.3%) and attending related conferences (n=96, 81.4%). ASP was considered as a contributing factor in 68.6% of the cases (n=81) (Table 6).
Table 6. Recent changes in prescribing habits and reasons for change (n=118)
| n (%) | |
|---|---|
| Perceived recent change in prescribing habits | 118 (74.7) |
| Factors of change (n=118) | |
| Greater consciousness regarding ATB resistance | 114 (96.6) |
| Infectious Disease consults | 103 (87.3) |
| Conferences | 96 (81.4) |
| Articles in medical literature | 90 (76.3) |
| Antimicrobial Stewardship Program | 81 (68.6) |
| Visiting speakers | 57 (48.3) |
| Fellow/resident approach to ATB prescribing | 55 (46.6) |
| Pharmacists approach to ATB prescribing | 52 (44.1) |
| Budget constraints | 42 (35.6) |
| Other factors | 12 (10.2) |
Barriers for the implementation of ASP components and their practice are represented in Table 7. High percentages were found for all barriers. Physicians' lack of compliance with hospital guidelines and ATB policies was seen as the most important barrier to initiating and sustaining an ASP (n=136, 86.1%), followed by the minimal support of the MoPH was (n=132, 83.5%) and the absence of regulation and of national guidelines (n=127, 80.4%).
Table 7. Barriers to initiating or sustaining an antimicrobial stewardship program (n=158)
| Barriers | n (%) |
|---|---|
| Physicians lack of compliance with hospital guidelines and antibiotic prescribing policies | 136 (86.1) |
| Minimal support of the MOPH and absence of regulation | 132 (83.5) |
| Absence of national approved guidelines | 127 (80.4) |
| Lack of training and education in antimicrobial use | 126 (79.7) |
| Lack of support from the medical staff | 120 (75.9) |
| Lack of leadership to promote antimicrobial stewardship | 107 (67.7) |
| Lack of financial incentives to the ID physician/ pharmacist to initiate the program | 107 (67.7) |
| Lack of support from administration or department heads | 104 (65.8) |
| Infectious disease physician shortage | 90 (57.0) |
| Insufficient evidence my hospital would benefit | 73 (46.2) |
MOPH: Ministry of Public Health
Our survey included an open question to allow the respondents to comment on ASPs. Many of the comments were supportive for ASPs and highlighted their importance. Others made recommendations such as using information technology in ASPs, having procalcitonin test available in hospitals' lab, improving the quality of microbiological tests, and having local and national committees as well as standardization. Many comments stressed on communication importance and educational needs on ASP and antibiotic use. Finally, the low interest of hospitals in the subject despite its importance and the need to have ID physician to support pharmacist in their interventions were also mentioned.
DISCUSSION
ASPs have been shown to optimize treatment and to reduce adverse events associated with antibiotic use. Multiple templates for ASP are available. The present study aimed to describe implemented ASPs, as well as physicians' attitudes and barriers to these programs.
Almost all respondents reported at least one implemented ASP component in their hospital. However, in a previous study conducted in 2014 by Abou Ghannam et al. assessing ASPs in 58 Lebanese hospitals, 65% stated having an antimicrobial management control program in place.15 This highlights the need for a formal assessment in all Lebanese hospitals to have a clearer idea about ASP practices.
Having ID physician or ID pharmacist rounding, audit and feedback, and antimicrobials restricted to ID consultants were the most cited ASP components. Similar to other studies, restrictive measures like time-sensitive Automatic Stop Order were less often reported. This could be explained by the fact that these are usually less used in newly established ASPs to prevent opposition linked to feeling a loss of autonomy.24,25
Lebanese accreditation standards require policies and procedures for antibiotic use in hospitals. Surprisingly, specific interventions for defined infections were present in only 50-60% of the responses. For comparison, 77% of the 4184 US surveyed hospitals had implemented facility specific treatment recommendations in 2014.26
Furthermore, 71% (n=112) of the study respondents reported that local antibiograms were developed and monitored by the hospital's laboratory. In the 2014 Lebanese study of Abou Ghannam et al., 83.9% (n=47) of the hospitals reported developing local antibiogram, but only 54.5% (n=30) communicated the local antibiogram to medical staff.15 Communicating local antibiogram with prescribing physicians was proven to potentially influence their antibiotics selection, and to encourage antibiogram-based treatments.27 This practice should thus be encouraged.
Participating physicians had in general positive attitudes towards ASP. This support to ASP has been demonstrated by various studies from Canada and Australia.19,27-29 In fact, time spent interacting with ASP team was seen as beneficial with only less than 5% of our respondents reporting it inefficient.
In contrast, ASPs were considered as affecting physician's autonomy by one third of the participants. A similar result was also found in another study conducted in a pediatric hospital in Australia where loss of autonomy and interference in decision-making were reported, and restrictive measures got less acceptability in a study in nine Dutch hospitals.29,30
Physicians showed positive attitudes towards local guidelines, they agreed that asking for approval for restricted drugs made them think more carefully about the choice of antibiotic, and they all favored the use of computerized approval systems. These results are in agreement with those found in Australian surveys and among physicians in Saudi Arabia.25,29,31 Adversely, third of the Australian survey respondents reported that the approval process was time-consuming and detracting from other clinical duties.29
Antibiotic rounds and prospective audit and feedback were rated as the most useful interaction methods; these were also the preferred strategies for all respondents of the Dutch study.30
As for the barriers, the need for educational programs was raised. In fact, regular educational programs on the appropriate use of antibiotics seemed to be lacking. Education was seen as one of the most important ASP interventions in the Dutch hospitals study.30
Another important barrier was physicians' lack of compliance with hospital guidelines and antibiotic prescribing policies. Other studies reported that potential opposition from prescribers is one of the top barriers to ASP implementation.24 Other barriers reported by our participants were the minimal support from the MoPH and the absence of national approved guidelines.
Physicians noticed changes in their antibiotic prescribing during the last two years. The most cited changes were the use of more targeted therapies and shorter treatment durations. Greater consciousness of the resistance problem was the most frequent reason for these changes, while ASP was ranked fifth. This shows the need for ASP to be more engaging in order to lead to a bigger impact in antibiotic use. ASP pharmacists were perceived as a prescribing change factor by only 44% of respondents. This low impact of hospital pharmacists in Lebanon is not surprising as the average ratio of hospital pharmacists to hospitals is rather shy (1.9). Further, hospital pharmacists' role is still dispensary-based rather than clinical.32 This is unfortunate since current CDC guidelines emphasize on pharmacy-driven interventions and on the importance of an ASP that is co-lead by pharmacists.12
Limitations
The primary limitation to this study was the low response rate. Physicians are considered a hard-to-reach population.16-18 The response rate being very low during the collection period, and after reviewing strategies on improving physicians' response rates to web-based surveys, different strategies were adopted to improve the response rate.16,33,34 Also, it was impossible to select only hospital-based physicians from the list provided by the order because this information was not available; the response rate is thus underestimated. Moreover, the study did not allow for the verification of the proportionate distribution of the physicians; further studies should be done on a more representative sample.
A selection bias is possible as non-responders could have refrained from participating due to lack of knowledge about the subject. Also, physicians who feel most strongly about ASPs may have been more likely to respond introducing respondent bias, with attitudes being positively skewed. Physicians working in institutions without ASPs might be under-represented; one must be cautious in the generalization of the findings.
CONCLUSIONS
ASPs are a relatively a new patient safety initiative aimed at optimizing antimicrobial therapy. Despite positive attitudes for ASP implementation, one third of respondents considered ASPs as affecting physicians' autonomy. Most respondents reported a lack of regular educational programs and the need for support from the medical staff. The minimal support of the MOPH and absence of regulation and of national guidelines were reported as barriers to ASPs, as well as ID physicians' shortage.
Based on the results of this study, implementing strategies that are less restrictive, led by ID physicians and pharmacists, focused on antibiotic rounds, and prospective audit and feedback, would be more successful. The programs should also incorporate an educational component. Further, providing clear national and in-hospital guidelines, monitoring susceptibility and resistance data, and analyzing prescribing trends should be encouraged. Additional efforts are needed from the MoPH.
Additional research with better representativeness is required, as the present study is a pilot study with a response rate of 4%.