Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista de Osteoporosis y Metabolismo Mineral
versión On-line ISSN 2173-2345versión impresa ISSN 1889-836X
Rev Osteoporos Metab Miner vol.5 no.2 Madrid abr./jun. 2013
https://dx.doi.org/10.4321/S1889-836X2013000200007
Biomechanics and bone (& II): Trials in different hierarchical levels of bone and alternative tools for the determination of bone strength
Biomecánica y hueso (y II): Ensayos en los distintos niveles jerárquicos del hueso y técnicas alternativas para la determinación de la resistencia ósea
Caeiro J.R.1,2, González P.3, Guede D.1,4
1 Red Temática de Investigación en Envejecimiento y Fragilidad (RETICEF) - Instituto de Salud Carlos III - Ministerio de Economía y Competitividad - Madrid
2 Servicio de Cirugía Ortopédica y Traumatología - Complejo Hospitalario Universitario de Santiago de Compostela - A Coruña
3 Grupo de Nuevos Materiales - Departamento de Física Aplicada - Universidad de Vigo - Pontevedra
4 Trabeculae - Empresa de Base Tecnológica, S.L. Ourense - España
SUMMARY
For a greater understanding of the mechanical properties of bone as a whole, it is first necessary to determine the behaviour of each of the components in an individual way at its corresponding structural level, as well as its overall involvement. This is the basis of the theory of hierarchical structure of bone, which involves its division into different structural levels. In this work we review, level by level, this hierarchical structure, reviewing the different mechanical trials which are applied to each of the structure. In addition, the methods for the determination of bone strength alternative to the classic mechanical trials are presented, which in recent years have been contributing significantly to the mechanical understanding of bone.
Key words: biomechanics, bone tissue, bone strength.
RESUMEN
Para una mayor comprensión de las propiedades mecánicas del hueso en conjunto, debe determinarse primero el comportamiento de cada uno de los componentes de forma individual en su nivel estructural correspondiente y su implicación a nivel global. Ésta es la base de la teoría de la estructuración jerárquica del hueso, que implica su división en varios niveles estructurales. Repasamos en este trabajo dicha estructuración jerárquica nivel a nivel, revisando los distintos ensayos mecánicos que se aplican a cada una de las estructuras. Por otro lado, se presentan los métodos para la determinación de la resistencia ósea alternativos a los ensayos mecánicos clásicos, que en los últimos años están contribuyendo significativamente al entendimiento mecánico del hueso.
Palabras clave: biomecánica, tejido óseo, resistencia ósea.
Introduction
Whatever the type of force to which bone is subjected in vitro the elastic modulus is always proportional to the bone mineral density (BMD), which means that the load necessary for the deformation of bone will be proportional to the degree of its mineralisation. However, bone with a very high BMD would imply a high degree of stiffness, which means it would be highly brittle. This shows that there are other factors in addition to its mass which influence the biomechanical efficacy of bone, such as the composition of bone tissue and its architectonic structure (macro- and microscopic), all these being grouped under the term bone quality. It has been estimated that the quantity of bone is responsible for 60 to 80% of its biomechanical strength, while the remaining 20-40% depends on bone quality, and it would therefore be a great mistake to underestimate its importance [1]. So it is vitally important to understand the contribution of each of the components of bone to its overall mechanical strength.
In the first section of this review [2] we provide an introduction to the field of biomechanics focussed on bone. We present the basic concepts of the issue and demonstrate the classic mechanical tests which have been used for some time in order to understand the mechanical properties of bone. However, in recent years advances in the field of biomechanics have gone much further, with mechanical strength of the different structural levels of bone being analysed separately, which is a great help in understanding the capacity of bone overall to support the loads to which it is subjected. In the second section we want to review the tests carried out at all structural levels. In addition, alternative techniques to the classical tests are presented which are increasingly used in the determination of bone strength.
The hierarchical structure of bone and its biomechanical properties
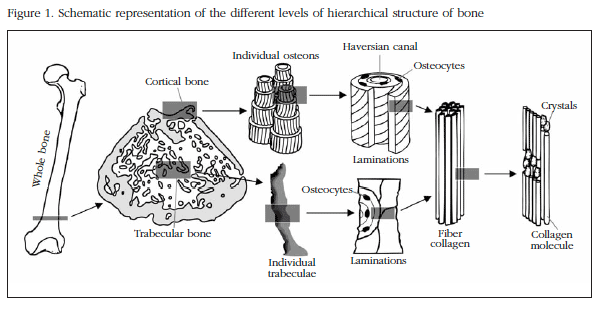
Bone is formed of an organic matrix composed mainly of type 1 collagen and a mineralised inorganic matrix (crystals of hydroxyapatite and calcium phosphatase). The collagen fibres which form bone are the result of the bonding by means of crossed links of a triple helix of chains of this material. This structure confers on bone its resistance to longitudinal traction and is largely responsible for its elasticity. The biomechanical properties which the collagen provides depend in turn on its utlrastructural characteristics, such as the quantity and orientation of its fibres or the stability of its links. In various pathological states these characteristics are seriously affected (mainly the stability of the links). On the other hand, the crystals are arranged in the spaces left in the organic matrix and are responsible for the stiffness of the bone and its resistance to compression, which means that these characteristics will be dependent on the quantity of the mineral, how densely it is packed and the arrangement of the crystals around the collagen fibres.
Due to its complex structure, in order to get to know and understand the biomechanical properties of bone its different structural levels need to be taken into account. Bone, in common with other biological materials, has what is known as a hierarchical structure composed of different levels as the scale varies. (Figure 1). These levels are defined in Table 1, according to the classifications established by different authors in recent years [3-6]. Each of these scales or hierarchical levels will have an influence on the biomechanical characteristics of bone.
Biomechanics of the whole bone
The mechanical behaviour of a material may be completely described by a group of material properties. However, the mechanical behaviour of a whole bone structure is much more complicated to predict, since it is the result of the material properties of each of its components and their geometric distribution in space.
Mechanical tests with whole bones or representative fractions of bone determine the properties of the bone as a whole, assuming that both the trabecular and cortical tissue can be modelled as a continuous structure, incorporating both its geometry and the properties of the materials of which it is composed. To be able to carry out this simplification, in doing so obviating the bone's anisotropy and heterogeneity, it is necessary that the test sample is significantly larger than the dimensions of its basic structural units. The biomechanical analysis of whole bone must always be accompanied by an analysis of its geometry. The mechanical behaviour of this type of sample is that which approximates most closely to the behaviour of bone in vivo; however, it is not appropriate to calculate material parameters at this level, since due to its complex geometry and the properties of the whole bone, it is not possible to identify changes in the microstructure or the extracellular matrix, which should be investigated at microscopic levels [5]. Nevertheless, tests of whole bone may be used to analyse the mechanical properties of the structural components, which is useful in the analysis of the effects which various factors, such as age, osteodegenerative diseases and their corresponding treatments, etc., provoke in the biomechanical properties of bone.
Much work has been carried out to understand the mechanical behaviour of whole bone, in which are used tests of compression and bending at three or four points and to a lesser extent, of torsion.
In the bending tests the measures consist of simple values of sagging and fracture loads, and stiffness (elastic zone incline). It is also possible to obtain a value for Young's modulus, but these calculations ignore the heterogeneity and the complex geometry of bone, since it is assumed that the bone is a perfect hollow tube, which means that the value obtained is simply approximated [7]. Nevertheless, it is the method most commonly used to estimate the mechanical properties of bone material in whole bone. Bone is more resistant to compression than to traction, and is even weaker in the face of shearing forces [8]. For example, when a long bone is loaded in a direction perpendicular to its longitudinal axis it suffers a bending load, since the impacted side is compression loaded, while the opposite side is traction loaded. As a result, the bone will begin to fail mechanically on the side opposite to the impact (the side subject to the traction), since this will reach its point of maximum resistance before the side subject to the compression.
Biomechanics of the tissue components
In terms of the structure of bone and its mechanical behaviour, we can see two subtypes of tissue: cortical bone and trabecular or spongy bone. The morphological differences between cortical and trabecular bone have significant biomechanical implications. The cortical bone has a higher elasticity modulus, which means that its stress-strain curve has a greater incline. This means that it is capable of supporting a greater degree of load per unit of surface area with a low strain index, which confers on it great stiffness. However, trabecular bone has a lower Young's modulus and biomechanically describes a flattened curve, which means that the supportable load per unit of surface area is lower, but with a higher strain index, which gives it greater flexibility (Figure 2).
Biomechanics of cortical or compact bone
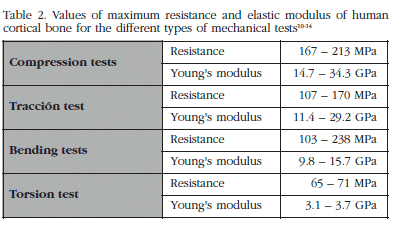
The biomechanical analysis of cortical bone is carried out on cubes or cylinders which contain a sufficient number of Haversian systems and interstitial spaces to be considered representative. The upper limit for sample size will be determined by the anatomical region from which it is extracted [9]. The mechanical properties of cortical bone depend on the type of test to which it is subjected. In Table 2 are shown the values for strength and elastic modulus for human cortical bone [10-14]. The variations in the values are due principally to the anatomical region from which they come and the age of the sample.
Although the reference test for determining the biomechanical properties of cortical bone is the traction test, what is most frequently used is the bending test. The resistance to traction is less than the resistance to compression, and in the torsion test the value for the Young's modulus is much less than in the other two cases. Due to the longitudinal orientation of the collagen fibres and the osteons, cortical bone has a greater resistance to the application of longitudinal (0o inclination) than transverse (90o inclination) loads, and for intermediate inclination values, intermediate values of resistance will be obtained. In addition, its biomechanical strength longitudinally is also greater than that found with torsion loads. While the properties of a whole long bone are a function of its tubular form and its density, those of isolated cortical bone depend on its density and the orientation of the osteons. Due to this fact, the resistance values for cortical bone make up 60% of the strength of whole bone, which implies a greater mechanical strength for this tissue component [15].
The density of cortical bone depends on its porosity and the mineralisation of its material, and in human bone it has a value of approximately 1.9 g/cm2, which is practically constant due to the fact that the cortical structure is quite compact [16]. It has been concluded that there is a positive correlation between cortical density and its biomechanical properties, such that if the former increases the latter improves. Porosity is defined as the relationship between bone volume and the total volume of the tissue, and is normally determined in a transverse section of cortical bone. Porosity and mineralisation explain 84% of the variation in stiffness in cortical bone [17], and experimental formulae have even been found which relate mineralisation with Young's modulus in such a way that an increase in mineralisation means a reduction in the elastic modulus [18].
The thickness and diameter of cortical bone are the main factors which affect its biomechanics. An increase in either of these characteristics results in an increase in bone strength. A long bone may be modelled as a cylindrical body, and, according to the basic laws of mechanics, resistance to the deformation of any cylindrical body subject to a force is directly proportional to its diameter. On the other hand the thickness of the cortical region and the quantity of bone mass are closely related, such that, with a constant bone mass, a variation in its distribution also modifies the bone's strength. The reduction in cortical thickness which happens with age, or in any osteodegenerative disease, has associated with it an increase in the risk of fracture.
Biomechanics of trabecular or spongy bone
In the case of trabecular bone the mechanical analysis is also performed using cubes or cylinders of this tissue subtype, of sufficient dimensions that the microstructural component does not influence the biomechanical properties. The structural properties of trabecular bone are usually determined using compression, traction or bending tests.
From the results obtained with these different tests it has been observed that trabecular bone, in the same way as with the cortical bone, has a greater resistance to compression than to any other type of load [19]. Its resistance in compression tests varies between 1.5 and 9.3 MPa, and the Young's modulus between 10 and 1,058 MPa, as a function of the region of the skeleton from which is comes. The density of human trabecular bone is approximately 0.43 g/cm2. It has been concluded from experiments that both its strength and Young's modulus are a function of the square of its density, such that a small increase in density produces large increases in the two aforementioned parameters [20].
The trabecular bone volumetric ratio (the quotient between the volume of trabecular bone and the total volume of the tissue, BV/TV) plays a very significant role in the mechanical stregth of bone. If the BV/TV reduces below 15% the structural integrity of the tissue is seriously endangered, having a much greater propensity to fracture. The number of trabeculae and their connectivity are also very significant in the biomechanical behaviour of spongy bone. The trabeculae are arranged vertically and horizontally, the latter arrangement being of vital importance to the bone's strength. It is possible to model spongy tissue as a combination of beams (horizontal trabeculae) and columns (vertical trabeculae), such that the former has the function of connection and securing the structure. A decrease in the number of trabeculae reduces strength, this reduction being more significant if it is the horizontal trabeculae which decrease. Reduced strength due to the narrowing of the trabeculae is reversible with the appropriate treatment. However, if the connectivity between the trabeculae disappears the loss of resistance becomes irreversible, since the original elasticity cannot be restored. Therefore, a structure with a greater number and thickness of, and connectivity between, trabeculae will be stronger than another with a lower number of trabeculae, less thick and with greater separation, even though both have the same bone mass.
The orientation of the trabeculae defines the degree of anisotropy. There is a correlation between the risk of fracture and the anisotropy of bone which is not dependent of the trabecular mass. The trabeculae are oriented such that they are stronger in the direction in which they normally support load, so resulting in heterogeneity or anisotropy in its structure. Therefore, if a region normally supports longitudinal loads (such as, for example, the femoral neck) its trabeculae are arranged geometrically so that it can better support these forces, and it is more resistant to loads in this direction (compression load), but there is a high risk of fracture with a load in another direction (for example, a transverse load due to a fall). Cortical bone also has an anisotropic behaviour due to the arrangements of the Haversian canals, but its mechanical impact is much less than is the case with spongy bone.
Biomechanics of osteons and individual trabeculae
The biomechanical analysis at this level describes the material properties of the tissue independently of it geometry, since it is carried out on samples small enough that the bone architecture does not have an influence on the result. In the case of cortical bone, the tests are carried out on a block of a few osteons and even only one, while for trabeculae bone a bundle of trabeculae would be used without its typical porous architecture, since in a sample of greater size the geometry would play a significant role in its biomechanical properties.
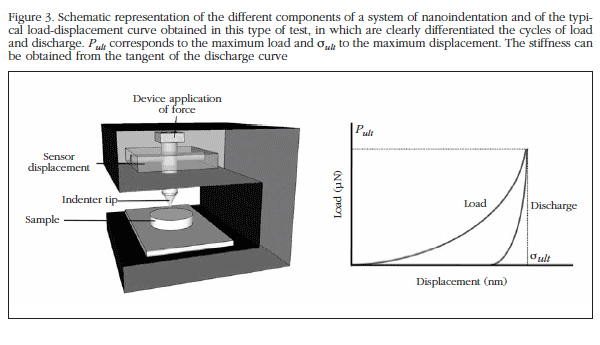
The use of nanoindentation tests for the analysis of the mechanical properties in very small samples has been developed in the last decade, allowing an in-depth analysis of structures such as trabeculae or individual osteons [21]. The technique of nanoindentation uses a rigid indentor with the aim of pressing on the surface of the material under test, thus provoking a local deformation of this surface. The force applied and the depth to which the indentor is applied is recorded both during the application of the force and once the sample is released, thus generating a load-displacement curve from which can be obtained the properties of the material (Figure 3).
The nanoindentation test equipment normally measures the force electromagnetically or electrostatically and the displacement by means of a capacitive sensor or a laser device. These methods allow the measurement of a force of between 1 and 500,000 µN, and a displacement of between 0.2 and 20,000 nm [22].
To perform an analysis of the osteons these first need to be isolated. Although it is possible to isolate a single individual osteon, their shape results in erroneous mechanical tests and the impossibility of comparing results. Therefore, the best option is to obtain, using a microtome which continually refrigerates the bone, a sample in a defined shape: a cylinder which best represents the properties of the osteons. The isolation of the osteons is a complex process, in which the orientation of the laminations, their mineralisation, the distance between the vascular canal and the external surface, etc., are taken into account [17].
The osteons in human bone may be classified as a function of the orientation of the collagen fibres in the layers of which they are composed. When the collagen fibres of all the layers which form the osteon are longitudinally oriented one refers to these as longitudinal osteons. If the fibres of one layer are longitudinally oriented and those of the adjacent layer transversally, one refers to these as alternate osteons. Much less common is a third type of osteon in which the collagen fibres are oriented transversally, which are called transverse or circular osteons. Tests of compression, traction, bending and torsion are used to study the mechanical properties of the osteons, in addition to the so-called pin test, commonly used in tubular material mechanics. The longitudinal layers better resist traction and torsion, while transverse layers offer better resistance to compression, bending and shearing loads. In addition, it has been confirmed that the distribution of the layers in the osteons of the long bones is not random, but that there is a high incidence of longitudinal layers in the parts of the bone which support traction loads, and a high incidence of transversal layers in the sections which principally support compression loads [23-34]. No effects of age, gender or body mass index have been found on the elastic modulus or the toughness of the layers [21,31,32,35], from which it may be deduced therefore that the elastic modulus and the toughness of the bone matrix is independent of these variables, which means that the reductions in the mechanical integrity of the whole bone could be due to other factors, such as changes in the mass and organisation of the tissues [31]. Most of the studies which analyse the mechanics of trabecular bone use samples of sufficient size such that the biomechanical properties are influenced by the trabecular architecture as well as by the material properties of the bone. Traditionally, trabecular bone is considered to be like a more porous cortical bone, with the assumption that it would have the same elastic modulus, but in reality, in order to understand the mechanical impact of trabecular tissue itself it is necessary to carry out tests with individual trabeculae. As in the case of individual osteons, the mechanical analysis of the trabeculae is a complicated process which even requires the design of specific equipment. Three point bending tests [36,37] and traction tests [38,39] have been carried out. In the last few years advances in computerised microtomography have allowed models of individual trabeculae to be obtained, which are subsequently analysed by means of finite elements analysis [40]. The result show that the Young's modulus of trabecular bone taken independently is considerably lower than that of cortical bone, probably due to the lower degree of organisation which the former displays. Recently, two research groups have independently analysed, using computerised microtomography, samples of human trabecular bone taken from different parts of the anatomy, carrying out a complete decomposition of the samples on individual plates and tubes and calculating their contribution to the elastic modulus by means of finite element analysis. The results obtained show a predominance of longitudinal plates and transverse tubes in the three anatomical zones, and that the axial loads on the trabecular bone are largely sustained by the trabecular volume axially aligned. In addition, it is suggested that the trabeculae in the form of plates dominate the overall elastic characteristics of trabecular bone [41-46].

Biomechanics of the bone molecular components
Bone at a molecular level is composed of proteins, glycoproteins and minerals, a composition which is known as an extracellular matrix. At this level it is interesting to study the mechanical properties of the collagen fibrils and the mineral components. The heterogeneity of the matrix makes the biomechanical analysis at this level yet more difficult, and the influence of the variations in the structure of the components is not known at present.
In 1997 Luo and collaborators presented a study in which the stiffness of the collagen molecules obtained from procollagen type 1 (which does not form intermolecular bonds) was measured using a system of optical tweezers and optical microscope [47]. Almost a decade later, using an electromechanical device, measures of resistance to traction, stiffness, and behaviour under fatigue of a collagen fibril were presented, while demonstrating for the first time its stress-strain curve [48]. A new experimental technique using atomic force microscopy and scanning electron microscopy have been used to manipulate and measure the mechanical properties of individual collagen fibrils in bone tissue. The stress-strain curve of the individual fibrils under traction stress shows an initial region of linear deformation for all the fibrils, followed by the non-homogeneous deformation above a critical deformation. This non-homogeneous deformation suggests possible changes in the mineral composition within each fibre [49].
The intrinsic mechanical properties of hydroxyapatite crystals have been determined by nanoindentation techniques. The basal faces of the crystals have a greater toughness and elasticity modulus than the lateral faces, but the latter are stronger. These results suggest that the crystals have a lower propensity to cracking and better resist microfractures on the lateral faces, which evidences the anisotropy of the hydroxyapatite crystals, which could have implications for the anisotropy observed on a larger scale [50].
The mechanics at a molecular level are influenced by all types of chemical interactions and unfortunately to date it has not been possible to carry out reliable and reproducible biomechanical tests at this level. The methods of in situ analysis which combine high resolution tools for structural determination, such as X-ray diffraction, with micromechanical tests are starting to provide information regarding the real deformation which takes place at the molecular level and at the level of the mineralised and non-mineralised collagen fibrils [51,52].
Biomechanical techniques alternative to classical tests
Qualitative ultrasound analysis (QUS)
It has been a while since ultrasound techniques started being used for the evaluation of the mechanical properties of bone [53,54]. These techniques present various advantages over the classical mechanical tests in the determination of the elastic properties of bone, since samples which are very small and of different shapes can be used. Although qualitative ultrasound analysis does not produce an image of the structure of the bone there is real evidence that the QUS measurements may provide information related to the structural organisation and material characteristics of the tissue [55]. The advantages of QUS lie in the fact that no exposure to radiation is involved, as well as it being carried out using relatively cheap and portable systems. On the other hand, its principle inconvenience is its lack of sensitivity, which means that is it currently relegated to being used as an auxiliary tool in the diagnosis of osteoporosis, which is subsequently confirmed using bone densitometry (DXA). However, it is very useful in research work [56-59].
Finite element analysis (FEA)
Mechanical analysis using numerical simulation, and specifically the finite element method, has become a tool of great value when studying the biomechanical response of bone under various load conditions. The first step in carrying out a finite element analysis is the acquisition of images of the anatomical area or bone sample, normally using computerised microtomography (CT) or nuclear magnetic resonance (MRI). The sets of images obtained are processed by means of complex algorithms and sophisticated software tools with the aim of obtaining a mesh or model of the finite elements of the selected volume of interest. With these models it is possible to carry out both a morphological analysis of the structure and a simulated biomechanical analysis which will provide data on the strength and Young's modulus of the object analysed [60]. The most common FEA is the static linear analysis which calculates the mechanical resistance to static loads (which do not vary with time) and which assume that the material is isotropic and homogeneous. However, the development in recent years of technologies which allow the acquisition of high resolution images of the bone (micro-CT, HR-MRI, etc.), along with the use of new algorithms which represent the structure of bone with greater precision, has allowed the creation of models with which loads on the tissue and their anisotropic elastic properties [61] can be calculated. The FEA provides ever more precise data, becoming a powerful tool for the understanding of the biomechanical behaviour of bone, and one of the most used in the last few years [62-64].
In 1998 the first device to carry out mechanical tests for compression and traction from within computerised microtomography equipment appeared, so that the test could be followed step by step through high resolution images [65]. The authors called this technique Image-Guided Failure Analysis (IGFA). IGFA is very useful in the biomechanical analysis of samples of trabecular bone since it allows the observation of the progression of a fracture, monitoring its initiation and its advance, while determining the influence of the microarchitecture of the sample, allowing knowledge of the microstructural properties local to the fractured areas as opposed to those areas remaining intact [66,67]. Recently a similar device has been developed to carry out torsion tests [68].
Nazarian and collaborators [69] concluded that 76% of the samples of trabecular bone from human lumbar vertebrae analysed with IGFA had minimum values of BV/TV, connective density and anisotropy in those regions in which mechanical failure had occurred with respect to the intact regions, with no significant differences being observed for other microstructural variables such as the number of trabeculae (Tb.N) thickness (Tb.Th) or trabecular separation (Tb.Sp), etc. On the other hand, our research group, in samples obtained from human osteoporotic femoral heads [70] found that the regions of fracture had worse values for all microstructural variables analysed, except for the degree of anisotropy. This reflects the fact that the region in which the failure occurs contains fewer trabeculae, of less thickness and which are less interconnected than the region which remains intact after the test, although in both the trabeculae are oriented in a similar way. In addition, in the region of the fracture tubular trabeculae are prevalent (theoretically less resistant to fracture) as opposed to trabeculae in plate form, which are more abundant in the intact region. The degree of correlation between σult and a linear combination of microstructural variables (BV/TV, Tb.Th and trabecular pattern factor Tb.Pf) improves significantly when, instead of using the average values for the whole structure, the values for the region in which the fracture originated are used.
Thanks to this technology it has been possible to observe the different mechanisms of fracture. So, when a compression force is applied to trabecular bone, the structures in plate form fail preferentially on bending, starting in an area of the plate already perforated. In the case of structures in bar form, buckling is the predominant form of collapse (manifested by significant transverse displacement in the main direction of compression).
Conclusions
The complex mineralised organic matrix which constitutes bone tissue is hierarchical, in different structural levels which will define the mechanical properties of bone. Each of these hierarchical levels contribute in a different way, and to a different extent, to the overall mechanical behaviour of bone, and this needs to be taken into account when studying its biomechanical properties.
Many studies are being carried out nowadays on the different structural levels, and every day there are greater advances in the understanding of the behaviour of each of these structures, both individually and when combined in the tissue as a whole. Techniques alternative to the classical mechanical tests are helping extensively in the execution of this objective. Among these alternative methods there are non-destructive techniques such as FEA and QUS, which permit the repetition of the test as many times as may be necessary, and the changing of variables as and when required, which opens up great possibilities in the field of biomechanics.
![]() Correspondence:
Correspondence:
David Guede
Trabeculae, S.L.
Parque Tecnolóxico de Galicia
Edificio "Tecnópole I" Local 22
32900 San Cibrao das Viñas
Ourense (España)
Correo electrónico:
dguede@trabeculae.com
Bibliography
1. Faulkner KG. Bone matters: Are density increases necessary to reduce fracture risk? J Bone Miner Res 2000;15:183-7. [ Links ]
2. Guede D, González P, Caeiro JR. Biomecánica y hueso (I): Conceptos básicos y ensayos mecánicos clásicos. Rev Osteoporos Metab Miner 2013;5(1):43-50. [ Links ]
3. Rho JY, Kuhn-Spearing L, Zioupos, P. Mechanical properties and the hierarchical structure of bone. Med Eng Phys 1998;20:92-102. [ Links ]
4. Weiner S, Wagner HD. The material bone: Structure mechanical function relations. Ann Rev Mater Sci 1998;28:271-98. [ Links ]
5. Hoffler CE, McCreadie BR, Smith EA, Goldstein SA. A hierarchical approach to exploring bone mechanical properties. En: An YH, Draughn RA, editors. Mechanical testing of bone and the bone-implant interface. CRC Press LLC (Boca Raton, USA) 2000;p.133-49. [ Links ]
6. An YH. Mechanical properties of bone. En: An YH, Draughn RA, editors. Mechanical testing of bone and the bone-implant interface. CRC Press LLC (Boca Raton, USA) 2000;p.41-63. [ Links ]
7. Sharir A, Barak MM, Shahar R. Whole bone mechanics and mechanical testing. Vet J 2008;177:8-17. [ Links ]
8. Turner CH. Bone strength: Current concepts. Ann NY Acad Sci 2006;1068:429-46. [ Links ]
9. Schaffler MB, Radin EL, Burr DB. Mechanical and morphological effects of strain rate on fatigue of compact bone. Bone 1989;10:207-14. [ Links ]
10. Reilly DT, Burnstein AH, Frankel VH. The elastic modulus of bone. J Biomech 1974;7:271-2. [ Links ]
11. Burnstein AH, Reilly DT, Martens M. Aging of bone tissue: Mechanical properties. J Bone Joint Surg Am 1976;58:82-6. [ Links ]
12. Cezayirlioglu H, Bahniuk E, Davy DT, Heiple KG. Anisotropic yield behavior of bone under combined axial force and torque. J Biomech 1985;18:61-9. [ Links ]
13. Keller TS, Mao Z, Spengler DM. Young's modulus, bending strength, and tissue physical properties of human compact bone. J Orthop Res 1990;8:592-603. [ Links ]
14. Cuppone M, Seedhom BB, Berry E, Ostell AE. The longitudinal Young's modulus of cortical bone in the midshaft of human femur and its correlation with CT scanning data. Calcif Tissue Int 2004;74:302-9. [ Links ]
15. Sedlin ED, Hirsch C. Factors affecting the determination of the physical properties of femoral cortical bone. Acta Orthop Scand 1966;37:29-48. [ Links ]
16. Ashman RB. Experimental techniques. En: Cowin SC, editor. Bone mechanics. CRC Press LLC (Boca Raton, USA) 1989;p.91. [ Links ]
17. Currey JD. The effects of drying and re-wetting on some mechanical properties of cortical bone. J Biomech 1988;21:439-41. [ Links ]
18. Schaffler MB, Burr DB. Stiffness of compact bone: Effects of porosity and density. J Biomech 1988;21:13-6. [ Links ]
19. Keaveny TM, Wachtel EF, Ford CM, Hayes WC. Differences between the tensile and compressive strengths of bovine tibial trabecular bone depend on modulus. J Biomech 1994;27:1137-46. [ Links ]
20. Hayes WC, Bouxsein ML. Biomechanics of cortical and trabecular bone: Implications for assessment of fracture risk. En: Mow VC, Hayes WC, editors. Basic Orthopaedic Biomechanics. Lippincott-Raven (Philadelphia, USA) 1997. [ Links ]
21. Rho JY, Zioupos P, Currey JD, Pharr GM. Variations in the individual thick lamellar properties within osteons by nanoindentation. Bone 1999;25:295-300. [ Links ]
22. VanLandingham MR. Review of instrumented indentation. J Res Natl Inst Stand Technol 2003;108:249-65. [ Links ]
23. Ascenzi A, Bonucci E. The tensile properties of single osteons. Anat Rec 1967;158;375-86. [ Links ]
24. Ascenzi A, Bonucci E. The compressive properties of single osteons. Anat Rec 1968;161:377-91. [ Links ]
25. Ascenzi A, Bonucci E. The shearing properties of single osteons 1972;172:499-510. [ Links ]
26. Ascenzi A, Bonucci E. Relationship between ultrastructure and "pin test" in osteons. Clin Orthop Relat Res 1976;121:275-94. [ Links ]
27. Frasca P, Harper RA, Katz JL. Strain and frequency dependence of shear storage modulus for human single osteons and cortical bone microsamples: Size and hydration effects. J Biomech 1981;14:679-81. [ Links ]
28. Ascenzi A, Baschieri P, Benvenuti A. The bending properties of single osteons. J Biomech 1990;23:763-71. [ Links ]
29. Lakes R. On the torsional properties of single osteons. J Biomech 1995;28:1409-10. [ Links ]
30. Zysset PK, Guo XE, Hoffler CE, Moore KE, Goldstein SA. Elastic modulus and hardness of cortical and trabecular bone lamellae measured by nanoindentation in the human femur. J Biomech 1999;32:1005-12. [ Links ]
31. Hoffler CE, Moore KE, Kozloff K, Zysset PK, Goldstein SA. Age, gender, and bone lamellae elastic moduli. J Orthop Res 2000;18:432-7. [ Links ]
32. Hoffler CE, Moore KE, Kozloff K, Zysset PK, Brown MB, Goldstein SA. Heterogeneity of bone lamellar-level elastic moduli. Bone 2000;26:603-9. [ Links ]
33. Hoffler CE, Guo XE, Zysset PK, Goldstein SA. An application of nanoindentation technique to measure bone tissue lamellae properties. J Biomech Eng-T Asme 2005;127:1046-53. [ Links ]
34. Ascenzi MG, di Comite M, Mitov P, Kabo JM. Hysteretic pinching of human secondary osteons subjected to torsion. J Biomech 2007;40:2619-27. [ Links ]
35. Rho JY, Zioupos P, Currey JD, Pharr GM. Microstructural elasticity and regional heterogeneity in human femoral bone of various ages examined by nano-indentation. J Biomech 2002;35:189-98. [ Links ]
36. Kuhn JL, Goldstein SA, Choi K, London M, Feldkamp LA, Matthews LS. Comparison of the trabecular and cortical tissue moduli from human iliac crests. J Orthop Res 1989;7:876-84. [ Links ]
37. Choi K, Kuhn JL, Ciarelli MJ, Goldstein SA. The elastic moduli of human subchondral, trabecular, and cortical bone tissue and the size-dependency of cortical bone modulus. J Biomech 1990;23:1103-13. [ Links ]
38. Ryan SD, Williams JL. Tensile testing of rodlike trabeculae excised from bovine femoral bone. J Biomech 1989;22:351-5. [ Links ]
39. Rho JY, Ashman RB, Turner CH. Young's modulus of trabecular and cortical bone material: Ultrasonic and microtensile measurements. J Biomech 1993;26:111-9. [ Links ]
40. Muller R, Ruegsegger P. Analysis of mechanical properties of cancellous bone under conditions of simulated bone atrophy. J Biomech 1996;29:1053-60. [ Links ]
41. Stauber M, Muller R. Age-related changes in trabecular bone microstructures: Global and local morphometry. Osteoporos Int 2006;17:616-26. [ Links ]
42. Stauber M, Muller R. Volumetric spatial decomposition of trabecular bone into rods and plates-a new method for local bone morphometry. Bone 2006;38:475-84. [ Links ]
43. Stauber M, Rapillard L, van Lenthe GH, Zysset P, Muller R. Importance of individual rods and plates in the assessment of bone quality and their contribution to bone stiffness. J Bone Miner Res 2006;21:586-95. [ Links ]
44. Liu XS, Saha PK, Wehrli FW, Sajda P, Guo XE. A 3D morphological analysis of trabecular bone based on individual trabeculae segmentation. Trans Orthop Res Soc 2006;31:1783. [ Links ]
45. Liu XS, Sajda P, Saha PK, Wehrli FW, Guo XE. Quantification of the roles of trabecular microarchitecture and trabecular type in determining the elastic modulus of human trabecular bone. J Bone Miner Res 2006;21:1608-17. [ Links ]
46. Liu XS, Sajda P, Saha PK, Wehrli FW, Bevill G, Keaveny TM, et al. Complete volumetric decomposition of individual trabecular plates and rods and its morphological correlations with anisotropic elastic moduli in human trabecular bone. J Bone Miner Res 2008;23:223-35. [ Links ]
47. Luo ZP, Bolander ME, An KN. A method for determination of stiffness of collagen molecules. Biochem Biophys Res Commun 1997;232:251-4. [ Links ]
48. Eppell SJ, Smith BN, Kahn H, Ballarini R. Nano measurements with micro-devices: Mechanical properties of hydrated collagen fibrils. J R Soc Interface 2006;3:117-21. [ Links ]
49. Hang F, Barber AH. Nano-mechanical properties of individual mineralized collagen fibrils from bone tissue. J R Soc Interface 2011;8:500-5. [ Links ]
50. Saber-Samandari S, Gross KA. Micromechanical properties of single crystal hydroxyapatite by nanoindentation. Acta Biomaterialia 2009;5:2206-12. [ Links ]
51. Grupta HS. Nanoscale deformation mechanisms in collagen. In: Fratzl P, ed. Collagen: Structure and mechanics. Springer (New York, USA) 2008;pp.155-73. [ Links ]
52. Buehler MJ. Hierarchical nanomechanics of collagen fibrils: Atomistic and molecular modeling. In: Fratzl P, ed. Collagen: Structure and mechanics. Springer (New York, USA) 2008;pp.175-247. [ Links ]
53. Yoon HS, Katz JL. Ultrasonic wave propagation in human cortical bone II: Measurements of elastic properties and microhardness. J Biomech 1976;9:459-62. [ Links ]
54. Ashman RB, Cowin SC, van Buskirk WC, Rice JC. A continuous wave technique for the measurement of the elastic properties of cortical bone. J Biomech 1984;17:349-61. [ Links ]
55. Pithioux M, Lasaygues P, Chabrand P. An alternative ultrasonic method for measuring the elastic properties of cortical bone. J Biomech 2002;35:961-8. [ Links ]
56. Nicholson PHF, Muller R, Lowet G, Cheng XG, Hildebrand T, Ruegsegger P, et al. Do quantitative ultrasound measurements reflect structure independently of density in human vertebral cancellous bone? Bone 1998;23:425-31. [ Links ]
57. Chaffai S, Peyrin F, Nuzzo S, Porcher R, Berger G, Laugier P. Ultrasonic characterization of human cancellous bone using transmission and backscatter measurements: Relationships to density and microstructure. Bone 2002;30:229-37. [ Links ]
58. Padilla F, Akrout L, Kolta S, Latremouille C, Roux C, Laugier P. In vitro ultrasound measurement at the human femur. Calcif Tissue Int 2004;75:421-30. [ Links ]
59. Muller M, Moilanen P, Bossy E, Nicholson P, Kilappa V, Timonen T, et al. Comparison of three ultrasonic axial transmission methods for bone assessment. Ultrasound Med Biol 2005;31:633-42. [ Links ]
60. Saxena R, Keller TS. Computer modeling for evaluating trabecular bone biomechanics. En: An YH, Draughn RA, editors. Mechanical testing of bone and the bone-implant interface. CRC Press LLC (Boca Raton, USA) 2000;pp.407-36. [ Links ]
61. Ulrich D, van Rietbergen B, Weinans H, Ruegseger P. Finite element analysis of trabecular bone structure: A comparison of image-based meshing techniques. J Biomech 1998;31:1187-92. [ Links ]
62. Bevill G, Eswaran SK, Gupta A, Papadopoulos P, Keaveny TM. Influence of bone volume fraction and architecture on computed large-deformation failure mechanisms in human trabecular bone. Bone 2006;39;1218-25. [ Links ]
63. Keaveny TM, Donley DW, Hoffmann PF, Mitlak BH, Glass EV, San Martin JA. Effects of teriparatide and alendronate on vertebral strength as assessed by finite element modeling of QCT scans in women with osteoporosis. J Bone Miner Res 2007;22:149-57. [ Links ]
64. Schileo E, Taddei F, Malandrino A, Cristofohni L, Viceconti M. Subject-specific finite element models can accurately predict strain levels in long bones. J Biomech 2007;40:2982-9. [ Links ]
65. Müller R, Gerber SC, Hayes WC. Micro-compression: a novel technique for the nondestructive assessment of local bone failure. Technol Health Care 1998;6:433-44. [ Links ]
66. Nazarian A, Müller R. Time-lapsed microstructural imaging of bone failure behavior. J Biomech 2004;37:55-65. [ Links ]
67. Nazarian A, Stauber M, Müller R. Design and implementation of a novel mechanical testing system for cellular solids. J Biomed Mater Res B - Appl Biomater 2005;73B:400-11. [ Links ]
68. Nazarian A, Bauernschmitt M, Eberle C, Meier D, Müller R, Snyder BD. Design and validation of a testing system to assess torsional cancellous bone failure in conjunction with time-lapsed micro-computed tomographic imaging. J Biomech 2008;41:3496-501. [ Links ]
69. Nazarian A, Stauber M, Zurakowski D, Snyder BD, Müller R. The interaction of microstructure and volume fraction in predicting failure in cancellous bone. Bone 2006;39:1196-202. [ Links ]
70. Guede D, Dapía S, Caeiro JR. Relación entre las propiedades biomecánicas y las variaciones locales en la microestructura ósea en cabeza femoral humana osteoporótica. Rev Osteoporos Metab Miner 2010;2(3):11. [ Links ]











 texto en
texto en