Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista de Osteoporosis y Metabolismo Mineral
versão On-line ISSN 2173-2345versão impressa ISSN 1889-836X
Rev Osteoporos Metab Miner vol.6 no.4 Madrid Nov./Dez. 2014
https://dx.doi.org/10.4321/S1889-836X2014000400005
Effect of spinal cord injury recently in bone turnover and in bone mass evolution of complete motor. Preliminary findings
Efecto de la lesión medular motora completa reciente en el recambio óseo y en la evolución de la masa ósea. Resultados preliminares
Gifre L.1, Vidal J.2, Ruiz-Gaspà S.3, Portell E.2, Monegal A.1, Muxi A.4, Guañabens N.1,3 and Peris P.1,3
1 Servicio de Reumatología - Unidad de Patología Metabólica Ósea - Hospital Clínico de Barcelona
2 Unidad de Lesionados Medulares - Instituto de Neurorrehabilitación Guttmann - Universidad Autónoma de Barcelona - Badalona
3 CIBERehd
4 Servicio de Medicina Nuclear - Hospital Clínico de Barcelona
Work scholarship from the SEIOMM to attend the 35th Congress of the ASBMR (Baltimore, 2013).
This work is funded by grants from the Clinic Hospital of Barcelona and the La Marató Foundation of TV3.
SUMMARY
Background and aim: Spinal cord injury (SCI) has been associated with a marked increase in bone loss and a higher incidence of skeletal fractures, however the pathogenesis and clinical management of this condition remains unclear. The aim of this study was to analyze the bone mineral density (BMD) evolution in patients with complete SCI and its relationship with parameters of bone metabolism and bone turnover markers.
Methods: Patients with a recent complete motor SCI (ASIA A)(<6 months) were prospectively included. Bone metabolism parameters (calcium, phosphate, PTH and 25-OHD), bone turnover markers (bone formation: procollagen type 1 aminoterminal propeptide -P1NP-, bone alkaline phosphatase -bone AP-, osteocalcin -OC-; bone resorption: C-telopeptides of type I collagen -CTx-) and BMD were assessed in all patients at baseline and at 6 months. The results were compared with a control group.
Results: 23 men with complete SCI (ASIA A) and a mean age of 38±15 years were included at 102±33 days of SCI onset. 52% had paraplegia. 12 patients were assessed at 6 months of follow-up. Patients with SCI showed a significant increase in bone turnover markers, especially P1NP and CTx, compared to controls (P1NP: 191±90 vs 51±19 ng/ml, p<0.001; CTx: 1.37±0.49 vs 0.51±0.23 ng/ml, p<0.001). At 6 months, bone turnover markers decreased (P1NP: -34%, p=0.005 and CTx: -26%, p=0.002) and BMD had a mean decrease of 12% at total femur (p=0.002) compared to baseline, with osteoporosis development in 50% of patients. Bone markers (bone AP, P1NP and OC) were negatively correlated with total femur BMD values.
Conclusions: Patients with complete SCI show a marked increase in bone turnover and bone loss, especially at the proximal femur, with the development of osteoporosis being observed in 50% of these patients at 6 months of follow-up. These findings indicate the need to implement preventive measures within the therapeutic approach in these patients.
Key words: osteoporosis, spinal cord injury, bone metabolism, bone turnover markers.
RESUMEN
Fundamento y objetivos: La lesión medular (LM) se asocia con una marcada pérdida de masa ósea y un aumento de la incidencia de fracturas; sin embargo, la etiopatogenia y el manejo clínico de estos pacientes es incierto. El objetivo de este estudio ha sido valorar la evolución de la densidad mineral ósea (DMO) en pacientes con LM reciente, y su relación los parámetros del metabolismo fosfocálcico y los marcadores de recambio óseo.
Métodos: Estudio prospectivo en pacientes con LM motora completa (ASIA A) reciente (<6 meses). En todos ellos se valoró: parámetros del metabolismo fosfocálcico (calcio, fosfato, PTH y 25-hidroxivitamina D), marcadores de recambio óseo (formación: propéptido aminoterminal del procolágeno tipo I -P1NP-, fosfatasa alcalina ósea -FA ósea-, osteocalcina -OC-; resorción: telopéptido carboxiterminal del colágeno tipo I -CTx-) y DMO, basal y a los 6 meses de seguimiento. Los resultados se compararon con un grupo control.
Resultados: Se incluyeron 23 varones con una LM severa (ASIA A), con una edad media de 38±15 años a los 102±33 días de la LM. El 52% tenía una paraplejia. 12 pacientes fueron valorados a los 6 meses de seguimiento. Tras la LM se observó un aumento significativo de los marcadores de recambio óseo, especialmente P1NP y CTx, comparado con el grupo control (P1NP: 191±90 vs. 51±19 ng/ml, p<0,001; CTx: 1,37±0,49 vs. 0,51±0,23 ng/ml, p<0,001). Los marcadores de recambio óseo disminuyeron a los 6 meses de seguimiento (P1NP: -34%, p=0,005 y CTx: -26%, p=0,002). Asimismo, a los 6 meses se observó una marcada disminución de la DMO en fémur proximal (-12% en fémur total, p=0,02) comparada con los valores basales, y el desarrollo de osteoporosis en el 50% de los pacientes. Se observó, además, una correlación negativa entre los valores de DMO en fémur total y los marcadores de recambio óseo (Fosfatasa alcalina ósea, P1NP y osteocalcina).
Conclusión: Tras la LM se produce un marcado aumento del recambio óseo y de la pérdida de masa ósea, especialmente en fémur proximal, que conduce al desarrollo de osteoporosis en el 50% de los pacientes, una complicación que ya se observa a los 6 meses de seguimiento, y que indica la necesidad de adoptar medidas preventivas en el abordaje terapéutico de estos pacientes.
Palabras clave: osteoporosis, lesión medular, metabolismo óseo, marcadores recambio óseo.
Introduction
An absence of mechanical load on the skeleton is associated with a marked loss of bone mass which may result in the development of osteoporosis and fractures. Spinal cord injury (SCI), especially when they are complete, are a common cause and an archetypal example of an absence of load on the skeleton. So, a marked loss of bone mineral density (BMD) after an SCI has been reported, of the order of 35% after two years from its occurrence, and the development of osteoporosis and fractures in more than 50% of patients1-5. Although the physiopathology of this process is not well understood, after an SCI there has also been observed a marked increase in bone turnover, especially during the first year after the SCI6-10. While the absence of load is the main factor related to this finding, the regulatory mechanism for this process is not clear. This fact, together with the absence of guidelines aimed at the prevention and treatment of osteoporosis after an SCI, could be the cause of defective treatment for these patients. Indeed, a study recently carried out in our unit found evidence that after a complete SCI fewer than 10% of patients had obtained anti-osteoporotic treatment, even after having had fragility fractures2, a fact which has also been observed in other studies11. It is important to remember that the individuals who have a complete SCI are usually young people, which means that the risk of developing fractures over their life time is very high, clearly increasing at 3-5 years after the SCI2, which indicates the necessity of adopting preventative measures in these patients.
The objective of this study is to analyse the development of BMD and bone turnover in patients with recent SCI, and the factors relating to the loss of bone mass in this process. This preliminary analysis shows the development of the BMD and the markers for bone turnover in the short-term, in the first 6 months of monitoring.
Patients and methods
Population of the study
Prospective study in which were included patients with recently occurring (<6 months) SCI of traumatic origin and severe in character (complete motor SCI [ASIA scale: A or B]). The patients were recruited consecutively (from August 2010 to January 2012) at the Guttman Institute for Neurorehabilitation and then referred to the bone metabolism pathology unit of the rheumatology service of the Clinical Hospital of Barcelona.
Those included were patients over 18 years of age, while those having diseases or processes which affected bone metabolism (Paget's disease of bone, rheumatoid arthritis, hyperparathyroidism, hypercortisolism, malabsorption syndrome, malignant tumours, transplants, recent pregnancy or breastfeeding) and/or who were following treatment with drugs which would interfere with bone metabolism (bisphosphonates, strontium ranelate, selective estrogen receptor modulators, calcitonin, hormone therapy, denosumab and teriparatide, amongst others) were excluded.
In all the patients the risk factors for osteoporosis were evaluated, including: family history of femoral fractures, personal history of fractures, tobacco and alcohol consumption, dietary intake of calcium (mg/day) and history of renal lithiasis. In addition the cause, level (tetraplegia/paraplegia), severity and type (spastic/flaccid) of SCI and associated complications were analysed.
The severity of the SCI was evaluated using the ASIA (American Spinal Injury Association) scale which, classifies MLs into 5 categories according to motor function and residual sensitivity: A: complete motor and sensory loss; B: complete motor and partial sensory loss; C and D: partial motor and sensory loss; E: without motor or sensory lesion12.
The results were compared with a healthy control group of the similar age and sex.
The study was carried out with the approval of ethics committee of the hospital and adjusted in accordance with directives pertaining to research in humans. All the patients signed their informed consent for inclusion.
Analytical tests
Blood was taken from all patients at between 8 and 10 in the morning after overnight fasting. A biochemistry profile was performed which included calcium, phosphate and creatinine, determined by standard techniques, and levels of 25-hydroxyvitamin D (25-OHD) and parathyroid hormone (PTH) were assessed using automated chemoluminescence (Liaison, Diasorin and Advia Centaur XP, Siemens, respectively). In addition, the following biochemical markers for bone formation were determined: bone alkaline phosphatase (Bone AP, IDS, Vitro); osteocalcin (OC, radioimmunoassay, Elsa-Osteo-Cis, Gif-sur-Yvette, France) and amino-terminal propeptide of collagen type 1 P1NP, Cobas e411 automated method, Roche), and for bone resorption: carboxyl-terminal telopeptide of collagen type 1 (CTx, Cobas e411 automated method, Roche).
Bone mineral density
The BMD in the lumbar spine, proximal femur (femoral neck and total femur) and in the lower limbs (EI) were determined in all patients by means of double X-ray absorptiometry (DXA; Lunar Prodigy, Radiation Corporation Madison, WI, US). The densitometric risk categories (normal BMD, osteopenia and osteoporosis were defined according to the criteria of the WHO13.
Statistical analysis
The results are expressed as the mean ± standard deviation of the mean (SD). The differences between the means of the continuous variables were analysed using the Mann-Whitney nonparametric U test, and the differences between proportions, by the Fisher test. For the comparison of paired variables the Wilcoxon test was used. To evaluate the association between variables the Pearson correlation coefficient was used. A value p<0.05 was considered statistically significant. The statistical analysis of the data was carried out using the SPSS program (version 18.0, Chicago, US).
Results
The clinical characteristics of the patients included in the study are shown in Table 1.

The study included 23 males with an average age of 38±15 years (range: 18-64) at an average of 102±33 days from suffering the SCI. All the patients had a severe SCI (ASIA A); 48% had tetraplegia and 52% paraplegia. The majority of patients (83%) had an SCI of the spastic type. All the patients had a severe residual functional affectation: 3 patients (13%) remained totally immobilized in bed, the rest (87%) required a wheelchair for mobility. The main cause of the SCI was a traffic accident (57%). The rest of the patients had an SCI due to falling (17%), diving into shallow water (13%), a sporting accident (9%) or domestic accident (4%). 12 of the 23 patients (7 with tetraplegia and 5 with paraplegia) were newly assessed after 6 months of follow up.
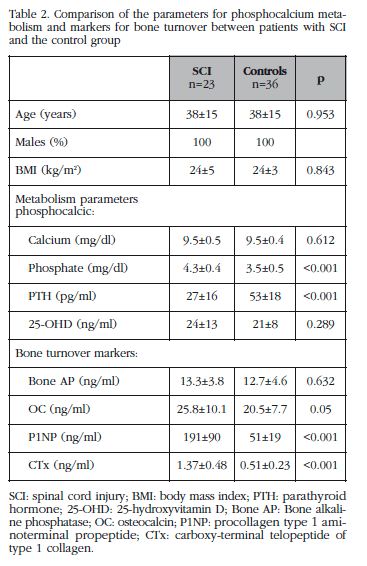
After the SCI a marked increase in markers for bone turnover (OC, P1NP and CTx) were observed compared with the control group (Table 2). No significant differences were observed in the value of bone markers as a function of the degree of lesion (patients with tetraplegia vs those with paraplegia). Also, those patients with SCI had a significant increase in levels of phosphate in the blood, and a reduction in values of PTH compared with the control group (Table 2). 39% of the patients had vitamin D deficit (<20 ng/ml), however, no differences were observed in levels of 25-OHD or in values of calcium in the blood compared with the control group.
At 6 months of follow up a significant reduction in markers for bone turnover (P1NP: -34%, p=0.005 and CTx: -26%, p=0.002) were observed, although they remained higher with respect to the control group, and there was a normalisation of the parameters for phosphorous-calcium metabolism (baseline phosphate: 4.4±0.4 mg/dl vs 3.9±0.4 mg/dl in follow up, p=0.011; baseline PTH: 32±20 pg/ml vs 40.8±22.9 pg/ml in follow up, p=0.09).
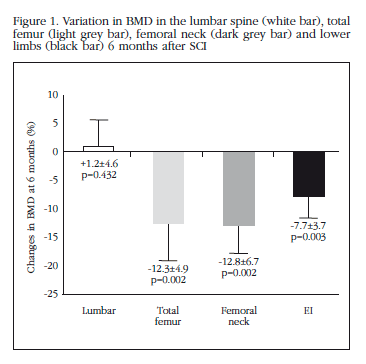
The BMD in the proximal femur and in the EI reduced significantly at 6 months follow up (total femur: -12.3+4.9%, p=0.002; femoral neck: -12.8+6.7%, p=0.002; EI: -7.7+3.7%, p=0.003) compared with baseline values (Figure 1). No significant changes were observed in the development of the lumbar BMD (1.214±0.2 g/cm2 baseline vs 1.224±.2 g/cm2 at 6 months, p=n.s). 50% of the patients had criteria for densitometric osteoporosis after 6 months follow up. No significant differences were observed in the development of BMD as a function of the level of SCI (tetraplegia vs paraplegia) or the type of lesion (spastic vs flaccid). None of the patients had skeletal fractures during the first 6 months of monitoring.
A negative correlation was observed between the values of BMD in the total femur and markers for bone turnover (Bone AP: r=-0.63, p=0.001; P1NP: r=-0.459, p=0.028; OC: r=-0.454, p=0.051). The values of CTx were not related to the values of BMD. The change in BMD at 6 months was not related to the change in markers for bone turnover.
Discussion
The results of this study show that after a complete motor SCI a marked increase in bone turnover and in loss of bone mass occurs, especially in the proximal femur, which leads to the development of osteoporosis in half of the patients, a complication which is already observed after 6 months of follow up, and which indicates the need to adopt preventative measures in the therapeutic approach with these patients.
Hence, over a period of only 6 months the patients included in this study had a loss of BMD in the proximal femur of 12% after an SCI and 50% developed densitometric osteoporosis. The BMD in the lumbar spine, however, remained stable during the follow up. These results coincide with those of earlier studies which indicate that the loss of bone mass after an SCI occurs early, already being evident at 6 weeks from the lesion1,14 and of greatest magnitude during the first two years after the SCI, with loss of BMD which varies between 8 and 35%, depending on it location and the time over which it had developed1,4,6,15,16, which leads to the development of osteoporosis and fractures in more than 50% of patients4. One of the main characteristics of the loss of bone mass associated with an SCI is its location, since, as seen in our study, it occurs below the level of the lesion, affecting, above all, the lower limbs1,17,18. This fact appears to be associated with the absence of mechanical load in the said location and which explains, furthermore, the high incidence of fractures in lower limbs which is observed in these patients, especially in the femur and tibia2,19,20. Also, even though it has been suggested that there is a greater loss of trabecular bone following an SCI15, other studies show that the bone loss occurring in this process takes place in various sections. Hence, a study which analysed the development of bone mass using peripheral quantitative computerised tomography (pQCT) in the proximal femur of patients with a recent SCI, describes cortical bone loss and an alteration in bone microarchitecture and strength after SCI, which would be measured by an increase in trabecular and endosteal bone resorption14. The results of our study also suggest an early affectation in both bone sections; thus, the magnitude of the bone loss in the different sections of the proximal femur, femoral neck and total femur, was similar after 6 months of follow up, of the order of 12% in both type of location. However, no significant changes were seen in the lumbar BMD after 6 months of follow up, a finding which has also been reported in other studies1,4, and which confirms the determining effect which the absence of mechanical load has in the lower limbs on bone loss associated with this process.
The majority of studies, both in experimental and human studies, report a marked increase in bone turnover after an SCI1,6.8-10, especially during the first year of the lesion, a finding which we also observed in our patients. In fact, the patients included in this study had an increase in markers for bone turnover, especially P1NP and CTx in the blood, after an SCI of the order of 2 to 3 times higher than the control group, which, in addition -as has been observed in the study of Sabour et al.21- are negatively correlated with values of BMD in the proximal femur. This increase in the markers for bone turnover diminishes progressively, such that at 6 months, although they remain slightly higher, their values have reduced significantly. Furthermore, in those patients it was also observed that there was a reduction in the values of PTH and a secondary increase in phosphate, two findings previously described in patients with recent SCI and which had been attributed to the marked increase in in bone turnover which occurred after the lesion6,8,9,22. Both parameters, PTH and phosphate, were normalised after 6 months follow up. Even though a high prevalence of hypovitaminosis D has been seen in patients affected by SCI23, this has been observed mostly in patients with a longstanding SCI. Our patients had recent MLs, less than 6 months earlier, a fact which may explain the absence of difference in values of vitamin D compared to the control group. Furthermore, although the increase in bone turnover which is observed after a recent SCI is a finding reported in most of the studies, the magnitude of this increase, and above all, its long-term development are aspects which are less well-documented. Hence, although the increase in turnover is particularly evident during the first few months after the SCI, some authors indicate the persistence of this increase some years after the SCI. Thus, Zehnder et al.6 observed that around 30% of patients had an increase in bone resorption, evaluated by determining levels of free deoxypyridinoline ten years on from the SCI. This is a fact which coincides with the persistence in BMD loss, although at a lower level, which patients have after several years with an SCI6,24, and which confirms the necessity of adopting preventative measures for the monitoring and treatment of this process.
The physiopathology of the alterations in bone turnover and loss of bone mass associated with an SCI is unclear, nevertheless, the absence of mechanical charge appears to be the determining factor for the loss of bone mass associated with this process1. Other factors such as the denervation which occurs after an SCI could also contribute as an additional factor in this loss. In this vein, experimental studies indicate a higher loss of bone in mice with an SCI than in mice subject to load on their extremities16,25.
One of the main limitations of this study is the loss of subjects during the follow up. However, this is an initial analysis which includes a highly homogeneous sample of patients, all having had a complete motor SCI in the past 6 months, which therefore allows the application of the results to other patients with similar characteristics. Furthermore, it is remarkable in featuring a prospective cohort which includes one of the highest numbers of patients with SCI.
In summary, the results of this preliminary study show that after a complete motor SCI there is a marked increase in bone turnover and loss of bone mass below the level of the lesion, which leads to the development of osteoporosis in half the patients during the first year of follow up. These results confirm the necessity of establishing preventative measures against the development of osteoporosis as part of the therapeutic approach taken with these patients.
Conflict of interest: There are no conflicts of interest on the part of the authors.
![]() Correspondence:
Correspondence:
Laia Gifre
Servicio de Reumatología
Hospital Clínic
Carrer Villarroel, 170
08036 Barcelona (Spain)
E-mail: lgifre@clinic.ub.es
Date of receipt: 11/07/2014
Date of acceptance: 24/10/2014
Bibliography
1. Jiang SD, Dai LY, Jiang LS. Osteoporosis after spinal cord injury. Osteoporos Int 2006;17:180-92. [ Links ]
2. Gifre L, Vidal J, Carrasco J, Portell E, Puig J, Monegal A, et al. Incidence of skeletal fractures after traumatic spinal cord injury: a 10-year follow-up study. Clin Rehabil 2014;28:361-9. [ Links ]
3. Lazo MG, Shirazi P, Sam M, Giobbie-Hurder A, Blacconiere MJ, Muppidi M. Osteoporosis and risk of fracture in men with spinal cord injury. Spinal Cord 2001;39:208-14. [ Links ]
4. Giangregorio L, McCartney N. Bone loss and muscle atrophy in spinal cord injury: epidemiology, fracture prediction, and rehabilitation strategies. J Spinal Cord Med 2006;29:489-500. [ Links ]
5. Bauman WA, Spungen AM, Wang J, Pierson RN Jr, Schwartz E. Continuous loss of bone during chronic immobilization: a monozygotic twin study. Osteoporos Int 1999;10:123-7. [ Links ]
6. Zehnder Y, Lüthi M, Michel D, Knecht H, Perrelet R, Neto I, et al. Long-term changes in bone metabolism, bone mineral density, quantitative ultrasound parameters, and fracture incidence after spinal cord injury: a cross-sectional observational study in 100 paraplegic men. Osteoporos Int 2004;15:180-9. [ Links ]
7. Jiang SD, Jiang LS, Dai LY. Changes in bone mass, bone structure, bone biochemical properties, and bone metabolism after spinal cord injury: a 6-month longitudinal study in growing rats. Calcif Tissue Int 2007;80:167-75. [ Links ]
8. Roberts D, Lee W, Cuneo RC, Wittamann J, Weard G, Flatman R, et al. Longitudinal study of bone turnover after acute spinal cord injury. J Clin Endocrinol Metab 1998;83:415-22. [ Links ]
9. Maïmoun L, Couret I, Mariano-Goulart D, Dupuy AM, Micallef JP, Peruchon E, et al. Changes in osteoprotegerin/RANKL system, bone mineral density, and bone biochemicals markers in patients with recent spinal cord injury. Calcif Tissue Int 2005;76:404-11. [ Links ]
10. Bubbear JS, Gall A, Middleton FRI, Ferguson-Pell M, Swaminathan R, Keen RW. Early treatment with zoledronic acid prevents bone loss at the hip following acute spinal cord injury. Osteoporos Int 2011;22:271-9. [ Links ]
11. Morse LR, Battaglino RA, Stolzmann KL, Hallett LD, Waddimba A, Gagnon D, et al. Osteoporotic fractures and hospitalization risk in chronic spinal cord injury. Osteoporos Int 2009;20:385-92. [ Links ]
12. Waring WP 3rd, Biering-Sorensen F, Burns S, Donovan W, Graves D, Jha A, et al. 2009 review and revisions of the international standards for the neurological classification of spinal cord injury. J Spinal Cord Med 2009;33:346-52. [ Links ]
13. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group, 843. World Health Organ Tech Rep Ser 1994;1-129. [ Links ]
14. Edwards WB, Schnitzer TJ, Troy KL. The mechanical consequence of actual bone loss and simulated bone recovery in acute spinal cord injury. Bone 2014;60:141-7. [ Links ]
15. Frey-Rindova P, de Bruin ED, Stüssi E, Dambacher MA, Dietz V. Bone mineral density in upper and lower extremities during 12 months after spinal cord injury measured by peripheral quantitative computed tomography. Spinal Cord 2000;38:26-32. [ Links ]
16. de Bruin ED, Dietz V, Dambacher MA, Stüssi E. Longitudinal changes in bone in men with spinal cord injury. Clin Rehabil 2000;14:145-52. [ Links ]
17. Liu D, Zhao CQ, Li H, Jiang SD, Jiang LS, Dai LY. Effects of spinal cord injury and hindlimb immobilization on sublesional and supralesional bones in young growing rats. Bone 2008;43:119-25. [ Links ]
18. Goemaere S, Van Laere M, De Neve P, Kaufman JM. Bone mineral status in paraplegic patients who do or do not perform standing. Osteoporos Int 1994;4:138-43. [ Links ]
19. Lala D, Craven BC, Thabane L, Papaioannou A, Adachi JD, Popovic MR, et al. Exploring the determinants of fracture risk among individuals with spinal cord injury. Osteoporos Int 2014;25:177-85. [ Links ]
20. Craven BC, Robertson CF, McGillivray CF, Adachi J.D. Detection and treatment of sublesional osteoporosis among patients with chronic spinal cord injury: proposed paradigms. Top Spinal Cord Inj Rehabil 2009;14:1-22. [ Links ]
21. Sabour H, Javidan AN, Latifi S, Larijani B, Shidfar F, Vafa MR, et al. Bone biomarkers in patients with chronic traumatic spinal cord injury. Spine J 2014;14:1132-8. [ Links ]
22. Maïmoun L, Fattal C, Micallef JP, Peruchon E, Rabischong P. Bone loss in spinal cord-injured patients: from physiopathology to therapy. Spinal Cord 2006;44:203-10. [ Links ]
23. Rivero González L, Méndez Suárez JL, Miranda Calderín G, Bárbara Bataller E, Sánchez Enríquez J, Sosa Henríquez M. Prevalencia de la hipovitaminosis D e hiperparatiroidismo secundario en la Unidad de Lesionados Medulares de Gran Canaria. Estudio preliminar. Rev Osteoporos Metab Miner 2013;5:67-72. [ Links ]
24. Garland DE, Adkins RH, Stewart CA. Five-year longitudinal bone evaluations in individuals with chronic complete spinal cord injury. J Spinal Cord Med 2008;31:543-50. [ Links ]
25. He JY .Jiang LS, Dai LY. The roles of the sympathetic nervous system in osteoporotic diseases: A review of experimental and clinical studies. Ageing Res Rev 2011;10:253-63. [ Links ]











 texto em
texto em