Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista de Osteoporosis y Metabolismo Mineral
versão On-line ISSN 2173-2345versão impressa ISSN 1889-836X
Rev Osteoporos Metab Miner vol.7 no.4 Madrid Nov./Dez. 2015
https://dx.doi.org/10.4321/S1889-836X2015000400005
Inappropriate use of proton-pump inhibitors and fragility fracture risk. A preliminary study
Uso inadecuado de inhibidores de la bomba de protones y riesgo de fractura por fragilidad. Estudio preliminar
Vera Rodríguez S.1, Martín Bethencourt E.1, Calvo Hernández L.M.2, Hernández Hernández D.1,2, Saavedra Santana P.1, Gómez de Tejada Romero M.J.1,3, Sosa Henríquez M.1,2
1 Instituto Universitario de Investigaciones Biomédicas y Sanitarias (IUIBS) - Grupo de Investigación en Osteoporosis y Metabolismo Mineral - Universidad de Las Palmas de Gran Canaria - Las Palmas de Gran Canaria (España)
2 Servicio Canario de la Salud - Hospital Universitario Insular - Unidad Metabólica Ósea - Las Palmas de Gran Canaria (España)
3 Departamento de Medicina - Universidad de Sevilla - Sevilla (España)
This study was partially funded by a research grant from the Canary Island Osteoporosis Society.
SUMMARY
Introduction: Proton-pump inhibitors (PPIs) are widely used drugs, though it should be noted that excessive use is not in line with the accepted indications in Spain and throughout Europe. Furthermore, some authors have established a possible PPI link to the risk of fracture. In this paper, we make an initial approach to knowledge into PPI consumption and analyze what indication is prescribed. We also studied the drugs' possible association with increased risk of fragility fracture in users.
Material and method: An observational, transversal, open and descriptive study in which a number of randomly-chosen patients were interviewed. These patients had been treated in outpatient, emergency and primary care centers. Some had also been treated in hospital wards.
Results: Of the 411 patients interviewed, 54% received PPIs. The average age was 63.3 years, compared with 46% that did not take them and who were younger presenting a mean age of 50.9 years. Gender distribution was similar. PPIs were mainly used as a "gastric protector", in 39.8% of the patients, with no indication appearing in the technical specifications for this group of drugs. Consumers of PPIs presented a higher prevalence of all fragility fractures.
Conclusions: More than half of the population surveyed consumed PPI. Of this group, about 40% did so without proper medical advice. Therefore, in addition to the higher prevalence of fragility fractures that suggest a possible increased risk of fracture among its users, we consider the need for a more rational use of these drugs. These preliminary findings point to a need for further studies to confirm the relationship between PPIs and the risk of osteoporotic fracture.
Key words: omeprazol, proton-pump inhibitors, abuse, side effects, osteoporosis, fracture.
RESUMEN
Introducción: Los inhibidores de la bomba de protones (IBP) son fármacos ampliamente utilizados, si bien esto conlleva a un sobreuso que no es acorde con las indicaciones aceptadas en España y en el resto de Europa. Por otro lado, algunos autores han establecido una posible implicación de los IBP en el riesgo de fractura. Con este trabajo hemos pretendido efectuar una primera aproximación al conocimiento del consumo de IBP en nuestro medio y analizar para qué indicación son prescritos, a la vez que estudiar su posible asociación con un mayor riesgo de fractura por fragilidad entre sus consumidores.
Material y método: Estudio observacional, transversal, abierto, descriptivo, en el que se entrevistó aleatoriamente a un número de pacientes que fueron atendidos en diferentes ámbitos sanitarios: consultas externas hospitalarias, servicios de urgencias, consulta de Atención Primaria y pacientes ingresados en planta hospitalaria.
Resultados: De los 411 pacientes entrevistados, el 54% de los pacientes recibían IBP, y cuya edad media era de 63,3 años, frente al 46% que no los tomaban y que eran más jóvenes, con una edad media de 50,9 años. La distribución por sexos fue similar. La principal razón de utilizar el IBP era como "protector gástrico", en el 39,8% de los pacientes, indicación no existente en la ficha técnica de este grupo de fármacos. Los consumidores de IBP tenían una mayor prevalencia de todas las fracturas por fragilidad.
Conclusiones: Más de la mitad de la población encuestada consume IBP, y de ella cerca del 40% sin una indicación médica correcta. Por esto, unido a la mayor prevalencia de fracturas por fragilidad que presentan -que nos hace pensar en un posible mayor riesgo de fractura entre sus usuarios- consideramos la necesidad de un uso más racional de estos fármacos. Estas conclusiones son preliminares pero, a la vista de estos resultados, creemos que puede ser interesante realizar más estudios dirigidos a comprobar de manera más firme la relación entre los IBP y el riesgo de fractura osteoporótica.
Palabras clave: omeprazol, inhibidores de la bomba de protones, abuso, efectos secundarios, osteoporosis, fractura.
Introduction
The proton-pump inhibitors (PPIs) are a group of drugs whose main action is a prolonged decrease in the stomach's hydrochloric acid secretion [1]. They are quite safe and widely used by the public, but not without side effects [2]. It has been reported that consumption of PPIs could be related to an increased risk of fragility fractures. There are published studies in the literature that support this [3-6] and others that deny it [7].
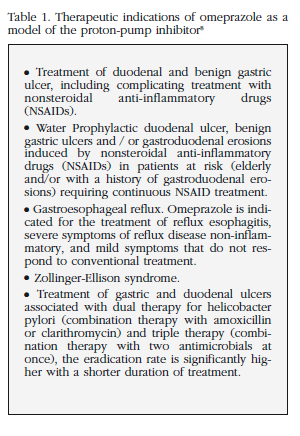
Moreover, PPIs have been used for many years as a stomach protector that may be caused by taking certain medications. This use is not stated in the therapeutic indications registered by the European Medicines Agency [8] and summarized in Table 1. There are no studies or scientific evidence to support its use for this purpose. Conversely, administration of PPIs in conjunction with other drugs may sometimes be counterproductive, as, for example, calcium carbonate, which requires an acid medium for optimal absorption [9], absorption would be inhibited, therefore, with simultaneous administration of PPI.
We conducted a study in a group of patients randomly recruited from different healthcare areas of the Insular University Hospital of Las Palmas, Spain, in order to collect initial data on the prevalence of PPI and the reasons why it is prescribed, and to study the prevalence of fragility fractures in these patients and possible links to PPI use.
Material and methods
To carry out this work, we designed a questionnaire composed of 10 items that were presented to a group of 411 randomly-chosen patients of both sexes. Those interviewed were treated in various health centers: emergency department, hospital internal medicine outpatient, primary care consultation and patients admitted to hospital on the wards. The minimum age for inclusion was 18 years, with no upper age limit. There was no choice in the type of patients in any working environment, whether in the hospital and health centers. Five doctors participated in the data collection. The questionnaire results were entered into a database designed ad hoc and consisting of a total of 20 items related, for the most part, to the use of PPIs.
The statistical study consisted of a descriptive analysis, using mean and standard deviation for the quantitative variables and percentage for categorical variables. To compare categorical variable tests Chi-square and Fisher were used. The Student t-test or Mann-Whitney U test were used to compare quantitative variables, depending on whether or not the variables followed a normal distribution. Variable normality was analyzed using the Kolmogorov-Smirnov. All results were adjusted for age. The significance level was set at 5% (p <0.05).
Results
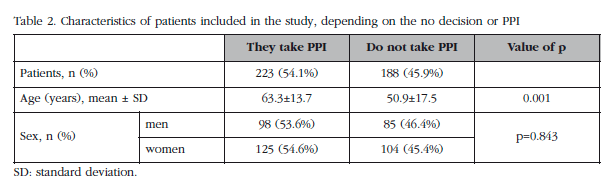
Table 2 shows the characteristics of the patients included in the study. A total of 411 patients were interviewed, more than half (54%) were receiving PPIs during the field-work survey period, while 46% did not take them. The average age of patients receiving PPIs was greater than those not receiving it (63.3 and 50.9 years, respectively). The age range was 18 to 95 years. The gender distribution of patients in both groups showed no statistically significant differences.
Table 3 shows the indications for which patients received PPIs. Overall, the most common indication was as proton pump inhibitors, which was obtained in about 40% of patients with hiatal hernia as a second cause, which was found in 10% of the users of PPIs. In this respect, no statistically significant differences between men and women were obtained.
The most commonly used PPIs were omeprazole (72.6%), followed by pantoprazole (13.4%). The use of lansoprazole, esomeprazole, and rabenprazol was more limited. The minimum length of treatment with PPIs was 1 month, which was observed in 8 cases, and the maximum 204 months, obtained in 2 patients.
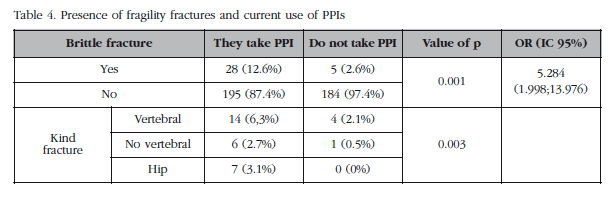
Table 4 shows the prevalence of fragility fractures in the participants. Patients taking PPIs at the time of the survey had a higher prevalence of fragility fractures than those not taking the drugs (12.6% vs 2.6% respectively), with an OR of 5,284, the difference being statistically significant. The prevalence of all different types, vertebral, non-vertebral and hip were higher in patients receiving PPIs (p=0.003).
Discussion
Our study shows that there is a significant consumption of PPIs among patients who may not be prescribed them. In our series, 54.1% of patients reported habitually taking PPIs at the time of the survey, similar to the results described elsewhere. In a population of elderly women in Australia, in the so-called Australian Longitudinal Study on Women's Health, with a sample size of 4,432 women, 52.5% received PPI4. In the hospital setting, in a sample of 834 admitted patients, 58.7% were taking PPIs, and "reviewing their indications" they were correct in only 50.1% of the patients [10]. In another study of hospitalized patients in a ward of respiratory diseases, 44% were receiving PPIs, of which 68% did not have a correct indication [11].
By far the most common reason for PPI use in our patients was as a stomach "protector" against other drugs (almost 40% of the total). We would point out that this indication does not exist in any PPI specifications sheet8, and there are no studies indicating that these drugs are effective for this. However, there is a widespread notion in the medical profession that it is prudent to administer various drugs, even when they may be gastro-erosive. The PPI should be added as a "protective" effect only described as effective and indication in the product information for these nonsteroidal anti-inflammatory drugs [8], and its usefulness is not proven in patients receiving oral corticosteroids.
Furthermore, although PPIs are considered safe with few side effects, their use has been associated with an increased prevalence of certain diseases, and the risk of acute myocardial [12], nephritis or hypomagnesemia [14] intersticial [13]. In the case of bone metabolic disorder IBP consumption has been associated with the presence of fractures in young adults [15], behaving as an independent risk factor for the production of fractures, both as a different studies [3-6,16] metaanalysis [17] where an increased risk of vertebral, non-vertebral and hip fractures, especially in the elderly was observed. This has caused concern among health authorities who have published several notices on this topic [17-20]. Our results are indicative in this sense, not conclusive, because, although the prevalence of fragility fractures was much higher in the group that received PPI, methodological factors discussed below make us cautious when considering the real meaning of these findings.
The study has several limitations. We do not consider the co-morbidity of these patients. Therefore, we cannot establish with certainty which users of PPIs have a greater risk of fragility fractures due to these drugs as with patients taking the drugs there could probably be others receiving drugs whose gastric effects were protected (although incorrectly) that would produce increased bone fragility (as in the case of corticosteroids). Furthermore, taking these other drugs may in turn indicate the existence of conditions that damage the bone (for example, rheumatoid arthritis). Another limitation is the small sample size. On the other hand, this is only a preliminary study to confirm the suspected overuse of PPIs, with an indication that there is no clinical evidence of any kind and that carries a huge unjustified economic cost. We should keep in mind the possible side effects described in other studies discussed above.
Regarding the economic costs, in 2014, more than 3 million containers of PPI were sold in the Canary Islands, which generated an expense of 20 million euros [20], much of which, as we have just shown, without a correct indication. This relates to consumption financed by the Canary Island Health Service. Actual consumption may be much higher, because PPIs are dispensed without a prescription.
In conclusion, although these results are preliminary and include a small sample size, our study shows that more than half of the patients receiving PPIs and, of these, almost 40% take it with an indication which is not approved, which could, in addition to a significant unnecessary health spending, generate an increased risk of other diseases, including fragility fractures. Therefore, we recommend more in depth, broader studies in this direction.
Competing interests
The authors report that none has any conflict of interest.
![]() Correspondence:
Correspondence:
Manuel Sosa Henríquez
c/Espronceda, 2
35005 Las Palmas de Gran Canaria (España)
Correo electrónico:
msosah@hotmail.com
Date of receipt: 31/10/2015
Date of acceptance: 26/11/2015
Bibliogragraphy
1. Hetzel DJ, Shearman DJ. Omeprazole inhibition of nocturnal gastric secretion in patients with duodenal ulcer. Br J Clin Pharmacol 1984;18:587-90. [ Links ]
2. Ali T, Roberts DN, Tierney WM. Long-term safety concerns with proton pump inhibitors. Am J Med 2009;122:896-903. [ Links ]
3. Yang S, Chen Q, Wei H, Zhang F, Yang DL, Shen Y, et al. Bone fracture and the interaction between bisphosphonates and proton pump inhibitors: a meta-analysis. Int J Clin Exp Med 2015;8:4899-910. [ Links ]
4. Van der Hoorn MM, Tett SE, de Vries OJ, Dobson AJ, Peeters GM. The effect of dose and type of proton pump inhibitor use on risk of fractures and osteoporosis treatment in older Australian women: A prospective cohort study. Bone 2015;81:675-82. [ Links ]
5. Ngamruengphong S, Leontiadis GI, Radhi S, Dentino A, Nugent K. Proton pump inhibitors and risk of fracture: a systematic review and meta-analysis of observational studies. Am J Gastroenterol 2011;106:1209-18. [ Links ]
6. Kwok CS, Yeong JK, Loke YK. Meta-analysis: risk of fractures with acid-suppressing medication. Bone 2011;48:768-76. [ Links ]
7. Targownik LE, Leslie WD, Davison KS, Goltzman D, Jamal SA, Kreiger N, et al. The relationship between proton pump inhibitor use and longitudinal change in bone mineral density: a population-based study (corrected) from the Canadian Multicentre Osteoporosis Study (CaMos). Am J Gastroenterol 2012; 107:1361-9. [ Links ]
8. Ficha técnica del omeprazol. Disponible en: http://www.aemps.gob.es/cima/pdfs/es/ft/68041/FT_68041.pdf consultado el 20-11-2015. [ Links ]
9. Straub DA. Calcium supplementation in clinical practice: a review of forms, doses, and indications. Nutr Clin Pract 2007;22:286-96. [ Links ]
10. Scagliarini R, Magnani E, Praticò A, Bocchini R, Sambo P, Pazzi P. Inadequate use of acid-suppressive therapy in hospitalized patients and its implications for general practice. Dig Dis Sci 2005;12:2307-11. [ Links ]
11. Niklasson A, Bajor A, Bergendal L, Simrén M, Strid H, Björnsson E. Overuse of acid suppressive therapy in hospitalised patients with pulmonary diseases. Respir Med 2003;97:1143-50. [ Links ]
12. Mayor S. People taking proton pump inhibitors may have increased risk of myocardial infarction, study shows. BMJ 2015;350:h3220. [ Links ]
13. Case Resaracho R, Jaio N, Vrotsoukanari K, Aguirre C. Case Report: Inadequate drug prescription and the rise in drug-induced acute tubulointerstitial nephritis incidence, NDT Plus 2010;555-7. [ Links ]
14. Zipursky J, Macdonald EM, Hollands S, Gomes T, Mamdani MM, Paterson JM, et al. Proton pump inhibitors and hospitalization with hypomagnesemia: A Population-Based Case-Control Study. PLOS Medicine 2014;11(9). [ Links ]
15. Freedberg DE, Haynes K, Denburg MR, Zemel BS, Leonard MB, Abrams JA, et al. Use of proton pump inhibitors is associated with fractures in young adults: a population-based study. Osteoporos Int 2015;26:2501-7. [ Links ]
16. Moberg LM, Nilsson PM, Samsioe G, Borgfeldt C. Use of proton pump inhibitors (PPI) and history of earlier fracture are independent risk factors for fracture in postmenopausal women. The WHILA study. Maturitas 2014;78:310-5. [ Links ]
17. Yu EW, Bauer SR, Bain PA, Bauer DC. Proton pump inhibitors and risk of fractures: a meta-analysis of 11 international studies. Am J Med 2011;124:519-26. [ Links ]
18. Servicio de uso racional del medicamento. Dirección General de Farmacia. Alerta de la FDA: IBP y aumento de riesgo de fracturas. Disponible en: www.gobiernodecanarias.eu/sanidad/scs/.../IBPyRiesgoDeFracturas.pdf. [ Links ]
19. MedWatch The FDA Safety Information and Adverse Event Reporting Program Proton Pump Inhibitors (PPI): Class Labeling Change. http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm213321.htm. consultado el 20-11-2015. [ Links ]
20. Bañón Morón N, Montes Gómez E, Alonso Rivero JM, Pérez Mendoza JM, Castellano Cabrera JL, De la Nuez Viera F. Bolcan. Boletín Canario de uso racional del medicamento del SCS. Vol 7. No1. Junio 2015. Disponible en: http://www3.gobiernodecanarias.org/sanidad/scs/ consultado el 20-11-2015. [ Links ]











 texto em
texto em