Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista de Osteoporosis y Metabolismo Mineral
versão On-line ISSN 2173-2345versão impressa ISSN 1889-836X
Rev Osteoporos Metab Miner vol.8 no.1 Madrid Jan./Mar. 2016
Changes induced by DKK1 in rheumatoid arthritis patients who commence biologic therapy treatment
Cambios inducidos en DKK1 en pacientes con artritis reumatoide que inician tratamiento con terapia biológica
Palma-Sánchez D.1, Haro-Martínez A.C.1, Gallardo Muñoz I.2, Portero de la Torre M.2, Mayor González M.1, Peñas E.1 and Reyes-García R.3
1 Unidad de Reumatología
2 Servicio de Radiología
3 Unidad de Endocrinología
Hospital General Universitario Rafael Méndez - Lorca - Murcia (España)
Work rewarded with the scholarship of Clinical Investigation FEIOMM 2013.
SUMMARY
Introduction: The aim of this study is to assess the relationship among inflammatory charge, cardiovascular risk and bone metabolism in patients with rheumatoid arthritis initiating biological therapy treatment.
Patients and methods: This is a prospective cohort study conducted in patients diagnosed with active rheumatoid arthritis (RA) assessed in the Rheumatology Unit and initiating biological therapy.
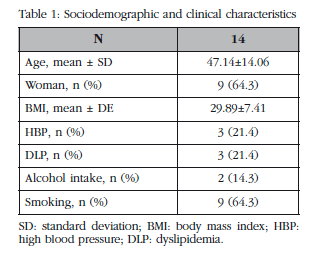
Patients will be selected consecutively, with preliminary data on 14 patients. We present preliminary data from 14 patients.
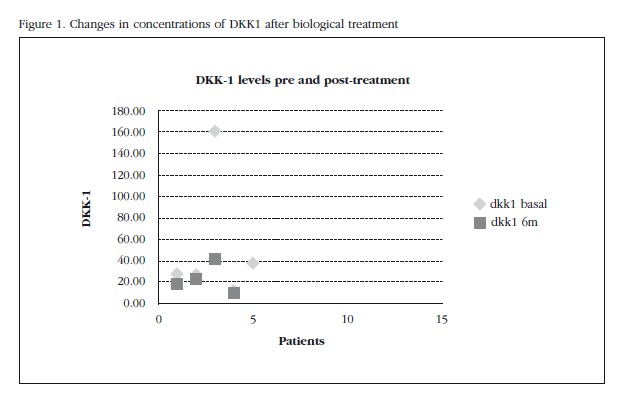
Results: Reduced Dickkopf-1 (DKK1) concentrations after commencing biological therapy were detected (baseline: 53.12±60.43 pg/ml vs 6 months 13.5±23.2 pg/ml, p=0.307) but without statistical significance. Changes were found in markers for bone remodeling with increased osteocalcin levels and CTX which were not statistically significant either.
Conclusions: We observed a nonsignificant decrease in DKK1 serum in patients with active RA treated with biologic therapy. Expanding the scope of study subjects and pending biochemical determinations will allow us, in the near future, to establish more precisely this link and the relationship of DKK1, bone remodeling, biological therapy and cardiovascular disease in RA patients.
Key words: rheumatoid arthritis, DKK1, biological therapy.
RESUMEN
Introducción: El objetivo del estudio es evaluar la relación entre la carga inflamatoria, el riesgo cardiovascular y el metabolismo óseo en pacientes con artritis reumatoide que inician tratamiento con terapia biológica.
Pacientes y métodos: Se trata de un estudio de cohortes prospectivo realizado en pacientes con diagnóstico de artritis reumatoide (AR) activa evaluados en la Unidad de Reumatología y que inician terapia biológica. Los pacientes serán seleccionados de forma consecutiva. Presentamos los datos preliminares de 14 pacientes.
Resultados: Encontramos una reducción en las concentraciones de Dickkopf-1 (DKK1) tras el inicio de la terapia biológica (basal: 53,12±60,43 pg/ml vs. 6 meses 23,2±13,5 pg/ml, p=0,307) pero no se alcanzó la significación estadística. Se encontraron cambios en los marcadores de remodelado con aumento en los niveles de osteocalcina y CTX que no alcanzó la significación estadística.
Conclusiones: En pacientes con AR activa tratados con terapia biológica hemos observado un descenso no significativo de las concentraciones séricas de DKK1. La ampliación tanto de los sujetos de estudio como de las determinaciones bioquímicas pendientes nos permitirán en un futuro próximo establecer de forma más precisa esta asociación, así como la relación entre DKK1, remodelado óseo, terapia biológica y enfermedad cardiovascular en pacientes con AR.
Palabras clave: artritis reumatoide, DKK1, terapia biológica.
Introduction
Rheumatoid arthritis (RA) and other inflammatory rheumatic diseases such as ankylosing spondylitis and psoriatic arthritis have an increased cardiovascular mortality due to accelerated development of atherosclerosis [1]. Persistent chronic inflammation and genetic factors have been associated with the development of accelerated atherosclerosis and consequently cardiovascular events [2].
The Wnt pathway has been involved not only in altering the bone metabolism [3] but also in cardiovascular disorders [4,5], which may be the common link between these diseases. The implication of rheumatoid arthritis in this way explained by pro-inflammatory cytokines involved in its pathogenesis, such as tumor necrosis factor alpha (TNF-α), which plays an important role in the process of osteoclast differentiation by increasing the ligand receptor activator of nuclear factor kB ligand (RANKL) and Dickkopf-1 (DKK1) and sclerostin, both Wnt pathway inhibitors [6]. Thus, control of activity in patients with RA should entail not only an increase in BMD but also reduced cardiovascular risk.
The aim of the study is to assess the relationship of the inflammatory burden, cardiovascular risk and bone metabolism in patients with rheumatoid arthritis who start treatment with biological therapy. To do this, we have analyzed the relationship between inflammatory activity, serum concentrations of antagonists of the Wnt pathway (DKK1), the specific cardiovascular disease by the modified SCORE method for RA, the intima-media carotid and bone disease in patients with RA, at the start of treatment with biological therapy and at 6 and 12 months of treatment. In this paper, preliminary data from the study are presented.
Patients and methods
This is a prospective cohort study of patients diagnosed with active RA evaluated at the Rheumatology Unit, and commence biological therapy. For the diagnosis of RA 1987 ACR criteria were used. Inclusion criteria were the following RA diagnosis, over 18 years of age, presence of disease activity (DAS> 2.4) despite treatment with synthetic disease-modifying drugs and signed informed consent. We excluded patients with previous cardiovascular events, previous osteoporotic fractures, osteoporosis different metabolic bone disease, chronic renal disease, chronic liver disease, type 1 and 2 diabetes mellitus, neoplastic disease, pregnancy and lactation.
The study was approved by the Ethics Committee for Clinical Research of the University Hospital Rafael Mendez. All participants were informed of the type of study and its procedures, and provided informed consent before any study procedure. The study was designed and conducted in accordance with the ethical standards of the Helsinki Declaration.
The following variables were collected: DKK-1 serum levels, sociodemographic characteristics, blood pressure (BP), DAS-28 VSG, visual analogue scale (VAS) of the patient's disease on a 0 to 10 scale, duration of the disease determined in years, response to treatment assessed by EULAR, values for rheumatoid factor and anti-citrullinated peptide antibodies, blood count, general biochemistry with hepatorenal function, lipid profile (total cholesterol, HDL, LDL, triglycerides), C-reactive protein (CRP ), and serum calcium, phosphorus parathyroid hormone (PTH), 25-hydroxyvitamin D3 (25-OH vitamin D3), bone turnover markers (bone alkaline phosphatase, osteocalcin, C-terminal telopeptide of type I collagen -u-CTX), thickness intima-media carotid (c-IMT), model SCORE (systematic coronary risk evaluation, systematic coronary risk Assessment) model modified for AR SCORE, and bone mineral density in the lumbar spine and hip measured by dual X-ray absorptiometry (DXA).
Biochemical determinations
The analysis of biochemical parameters was carried out by standard techniques.
Calciotropic hormone concentrations were determined by HPLC for 25-OH vitamin D3 and Elecsys systems for intact PTH. Remodeling biochemical markers were determined in an automated program (Roche Elecsys 2010).
DKK1 concentrations were evaluated by ELISA (Biomedica Medizinprodukte GmbH and Co. KG, Vienna, Austria) following manufacturer's instructions. For all other pending biochemical determinations, frozen samples at -80o C were used.
BMD Evaluation
Bone mineral density at the lumbar spine and femoral neck was assessed by dual X-ray densitometry (DXA) (Norland XR-800).
For postmenopausal women and men >50 years, the T-score was used to classify the central DXA in normal, osteopenia and osteoporosis. Z-score was used in the other cases; a Z-score <-2 was considered low bone mass.
Evaluation of c-IMT
The ultrasound evaluation of the carotid arteries was performed using Doppler ultrasound (Philips iU22) with a 9-3 MHz linear probe. The c-IMT was assessed and also the existence of plates. The c-IMT was measured in the distal third of both carotid arteries 1 cm above the bulb. The plate was defined as a greater focal thickening of 0.5mm within the arterial lumen or thickening >50% of the thickness of the adjacent intima or intimal thickness >1.5 mm.
Cardiovascular risk assessment
The patients' cardiovascular risk was determined by the SCORE model and modified to the AR SCORE. Those patients who had carotid ultrasound plates and/or c-IMT >0.9 were classified as patients at very high cardiovascular risk regardless of the SCORE obtained.
Statistical analysis
Data for continuous variables are expressed as mean ± standard deviation. The data for categorical variables are presented as percentages. Changes in quantitative variables before and after treatment were compared with Student's t test for paired samples. Categorical variables were compared by chi-square test.
Correlation analyzes were performed using Pearson's correlation (normal distribution) or Spearman (non-normal distribution). P values <0.05 were considered significant. For statistical analysis, the SPSS version 18.0 software (SPSS, Chicago, IL) was used.
Results
As of September 2015 14 naïve patients have been included for biological therapy. In this paper, we present the results at 6 months.
Demographic-clinical variables
The average age of the 14 patients was 47±14 years. 64.3% were women. 21.4% of the patients were hypertensive and 64.3% were smokers. The values of other variables are shown in Table 1.
Variables related disease
The average disease duration was 68±71 months (CI 10-240). The DAS 28-ESR mean baseline was 4.41±1, the average number of swollen joints 3±2, the average number of painful joints 4±3 and visual analogue scale (VAS) of the specific disease by 7±2 patient.
71.5% had positive rheumatoid factor and anti-citrullinated peptide antibodies. 64.3% of patients included in the study were taking disease modifying drugs (DMARDs) associated with biological therapy. The average prednisone dose was 3.7±2.5 mg. Only 44.4% of the patients had EULAR response to treatment at 6 months.
Analytical and variables related to bone metabolism
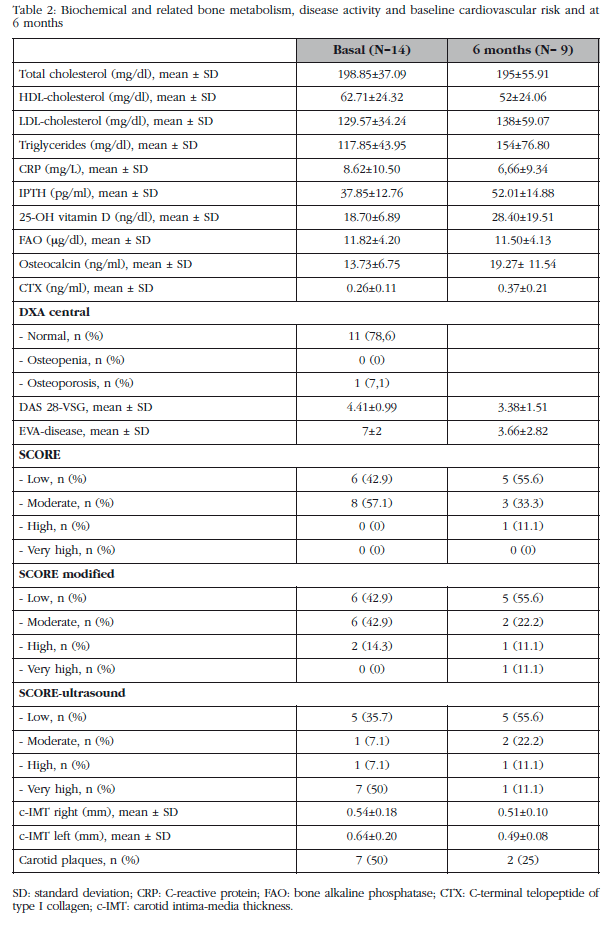
The values of these variables are represented in Table 2.
Correlation between bone remodeling, DKK1, disease activity and c-IMT
No significant correlation was found between disease activity measurement by DAS 28-ESR and levels of alkaline phosphatase, osteocalcin, CTX or DKK1. Nor did we observe any relationship between markers of remodeling or concentrations of DKK1 and intima-media thickness.
We found no association between DAS28-ESR-measured disease activity and cardiovascular risk assessed by SCORE and modified SCORE.
DKK1 changes after treatment and relationship with disease parameters
We found decreased levels of DKK1 after commencing biological therapy (baseline: 53.12±60.43 pg/ml vs 6 months 23.2±13.5 pg/ml, p=0.307) which was not statistically significant (Figure 1). No statistically significant association between decreased levels of DKK-1 and EULAR response to treatment was detected. As for bone remodeling markers, insignificant increased osteocalcin levels and CTX were detected.
Discussion
Epidemiological studies have shown an association between BMD loss and cardiovascular calcification, morbidity and mortality [7-9]. The Wnt pathway is involved in regulating vascular calcification and differentiation of smooth muscle cells to osteoblasts10. It has been shown to increase the DKK1 expression [11,12] in carotid atherosclerotic plaques and increased serum concentrations of sclerostin in patients with atherosclerotic disease and type 2 diabetes [13].
Furthermore, elevated levels of circulating DKK1 in patients with RA have been shown, linked to radiological damage [14-18], and sclerostin expression seems to correlate positively with levels of DKK1 [19].
Our study showed a decrease in the levels of DKK-1 at 6 months of treatment, which is consistent with recently published by Briot, et al. [20]. In this article, patients with active RA treated with tocilizumab experienced a decrease in DKK1 concentrations and a decrease in formation markers. However, in our study we found an increase in markers of formation and post-treatment resorption, but this did not reach statistical significance. Our limited sample size has certainly influenced our results.
In conclusion, we can say that in patients with active RA treated with biological therapy we have observed a nonsignificant decrease in serum concentrations of DKK1 and a significant increase in bone resorption. Expanding both the number of study subjects as well as more pending biochemical determinations would allow us in the near future to more precisely establish this association, and also the relationship between DKK1, bone remodeling, biological therapy and cardiovascular disease in patients with RA.
Competing interests
The authors declare no conflicts of interest regarding this article.
![]() Correspondence:
Correspondence:
Rebeca Reyes García
Ctra. Nacional 340, Km 589
30800 Lorca - Murcia (España)
Correo electrónico: rebeca.reyes.garcia@gmail.com
Date of receipt: 08/10/2015
Date of acceptance: 06/02/2016
Bibliography
1. González-Gay M, González-Juanatey C, Martin J. Rheumatoid arthritis: A disease associated with accelerated atherogenesis. Semin Arthritis Rheum 2005;35:8-17. [ Links ]
2. González-Gay M, González-Juanatey C, López-Díaz MJ, Piñeiro A, García-Porrua C, Miranda-Filloy JA, et al. HLA-DRB1 and persistent chronic inflammation contribute to cardiovascular events and cardiovascular mortality in patients with rheumatoid arthritis. Arthritis Rheum 2007;57:125-32. [ Links ]
3. Baron R, Rawadi G. Targeting the Wnt/beta-catenin pathway to regulate bone formation in the adult skeleton. Endocrinology 2007;148:2635-43. [ Links ]
4. Towler DA, Shao JS, Cheng SL, Pingsterhaus JM, Loewy OP. Osteogenic regulation of vascular calcification. Ann NY Acad Sci 2006;1068:327-33. [ Links ]
5. Shao JS, Cheng SL, Pingsterhaus JM, Charlton-Kachigian N, Loewy OP, Towler DA. Msx2 promotes cardiovascular calcification by activating paracrine Wnt signals. J Clin Invest 2005;115:1210-20. [ Links ]
6. Walsh C, Gravallese EM. Bone loss in inflammatory arthritis: mechanisms and treatment strategies. Curr Opin Rheumatol 2004;16:419-27. [ Links ]
7. Tanko LB, Christiansen C, Cox DA, Geiger MJ, Mc Nabb MA, Cummings SR. Relationship between osteoporosis and cardiovascular disease in postmenopausal women. J Bone Miner Res 2005;20:1912-20. [ Links ]
8. Hyder JA, Allison MA, Wong N, Papa A, Lang TF, Sirlin C, et al. Association of coronary artery and aortic calcium with lumbar bone density: the MESA Abdominal Aortic Calcium Study. Am J Epidemiol 2009;169:186-94. [ Links ]
9. Naves M, Rodríguez-García M, Díaz-Lopez JB, Gómez-Alonso C, Cannata-Andia JB. Progression of vascular calcifications is associated with greater bone loss and increased bone fractures. Osteopor Int 2008;19:1161-6. [ Links ]
10. Tsaousi A, Mill C, George SJ. The Wnt pathways in vascular disease: lesson from vascular development. Curr Opin Lipidol 2011;22(5):350-7. [ Links ]
11. Towler DA, Shao JS, Cheng SL, Pingsterhaus JM, Loewy AP. Osteogenic regulation of vascular calcification. Ann N Y Acad Sci 2006;1068:327-33. [ Links ]
12. Ueland T, Otterdal K, Lekva T, et al. Dickkopf-1 enhances inflammatory interaction between platelets and endothelial cells and shows increased expression in atherosclerosis. Arterioscler Thromb Vasc Biol 2009;29:1228-34. [ Links ]
13. Morales-Santana S, García-Fontana B, García-Martín A, Rozas-Moreno P, García-Salcedo JA, Reyes-García R, Muñoz-Torres M. Atherosclerotic disease in type 2 diabetes is associated with an increase in sclerostin levels. Diabetes Care 2013;36:1667-74. [ Links ]
14. Voorzanger-Rousselot N, Ben-Tabassi NC, Garnero P. Opposite relationships between circulating DKK1 and cartilage breakdown in patients with rheumatoid arthritis amd Knee osteoarthritis. Ann Rheum Dis 2009;68:1513-4. [ Links ]
15. Diarra D, Stolina M, Polzer K, Zwerina J, Ominsky MS, Dwyer D, et al. Dickkopf-1 is a master regulator of joint remodeling. Nat Mes 2007;13:156-63. [ Links ]
16. Wang SY, Liu YY, Ye H, Guo JP, Li R, Liu X, et al. Circulation Dickkopf-1 is correlated with bone erosion and inflammation un rheumatoid arthritis. J Rheumatol 2011;38:821-7. [ Links ]
17. Liu YY, Long L, Wang SY, Guo JP, Ye H, Cui LF, et al. Circulation Dickkopf-1 and osteoprotegerin in patients with early and longstanding rheumatoid arthritis. Chin Med J (Eng) 2010;123:1407-12. [ Links ]
18. Garnero P, Tabassi NC, Voorzanger-Rousselot N. Circulating dickkopf-1 and radiological progression in patients with early rheumatoid arthritis treated with etanercept. J Rheumatol 2008;35:2313-15. [ Links ]
19. Heiland GR, Zwerina K, BaumW, Kireva T, Distler JH, Grisanti M, et al. Neutralisation of DKK1 protects from systemic bone loss during inflammation and reduces sclerostin espression. Ann Rheum Dis 2010;69:2152-9. [ Links ]
20. Briot K, Rouanet S, Schaeverbeke T, Etchepare F, Gaudin P, Perdriger A, et al. The effect of tocilizumab on bone mineral density, serum levels of Dickkopf-1 and bone remodeling markers in patients with rheumatoid arthritis. Joint Bone Spine 2015;82:109-15. [ Links ]











 texto em
texto em