My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista de Osteoporosis y Metabolismo Mineral
On-line version ISSN 2173-2345Print version ISSN 1889-836X
Rev Osteoporos Metab Miner vol.8 n.2 Madrid Apr./Jun. 2016
Study of the microstructure of femoral patients with hip osteoarthritis and hip fracture by microCT
Estudio de la microestructura femoral de pacientes con coxartrosis y con fractura de cadera mediante micro-TAC
Sainz-Aja Guerra J.A.1, Alonso M.A.2, Ferreño Blanco D.1, Pérez-Núñez M.I.2, Ruiz Martínez E.1, García-Ibarbia C.3, Casado del Prado J.A.1, Gutiérrez-Solana F.1 and Riancho J.A.3
1 Departamento de Ciencia e Ingeniería del Terreno y de los Materiales - LADICIM - ETS de Ingenieros de Caminos, Canales y Puertos - Universidad de Cantabria - Santander (España)
2 Departamento de Traumatología y Cirugía Ortopédica - Hospital U.M. Valdecilla - Universidad de Cantabria - Santander (España)
3 Departamento de Medicina Interna - Hospital U.M. Valdecilla - Universidad de Cantabria - IDIVAL - Santander (España)
SUMMARY
Whereas bone mineral density (BMD) is characteristically low in osteoporosis, it has been postulated that in osteoarthritis BMD is increased. We aimed to check this concept by analyzing bone volumen and structure in the femoral heads of patients with hip fractures (n=10) and with hip osteoarthritis (n=9). Unexpectedly, the analysis of microstructural parameters by microCT did not reveal significant differences between both groups. In addition, we did not find a significant decline in the trabecular bone volume across the age range studied. These results suggest that the evolution of the trabecular bone of the femoral head is different from the age-related decrease of bone mass in other regions of the skeleton. Elucidating the mechanism involved could suggest new approaches to treat the bone loss associated with aging.
Key words: hip fracture, osteoarthritis, microCT, microstructure.
RESUMEN
La disminución de la densidad mineral ósea (DMO), es decir, del volumen de tejido óseo por unidad de volumen del esqueleto, es característica de la osteoporosis, mientras que se ha sugerido que la artrosis se acompaña de un aumento de la DMO a nivel local y sistémico. Para comprobar esta hipótesis analizamos mediante microTAC el hueso trabecular de la cabeza femoral de 10 pacientes con fractura de cadera y 9 con coxartrosis. El análisis no reveló diferencias significativas entre ambos grupos en el volumen de tejido óseo trabecular, ni en los demás parámetros estructurales analizados. Tampoco se encontró una caída significativa del volumen de hueso trabecular con la edad. Esto indica que el hueso de esta región tiene una evolución peculiar. Los mecanismos responsables de ese comportamiento son desconocidos, pero su esclarecimiento podría, quizás, abrir la puerta a nuevos abordajes en el tratamiento de la pérdida de hueso asociada al envejecimiento.
Palabras clave: fractura de cadera, artrosis, microTAC, microestructura.
Introduction
Osteoporosis and arthrosis are very common disorders. Their manifestations include lower extremity involvement which is particularly significant. Thus, hip fractures and hip osteoarthritis are important because they may limit patients' quality of life. This often requires replacing the hip joint, a procedure that bears a significant burden on health services and takes up much orthopedic surgery and traumatology resources.
However, the pathogenesis of these processes is different. Osteoporotic fractures are the result of decreased bone strength, due to aging and other factors which cause a loss in structural mass and competence. Conversely, osteoarthritis reflects an alteration of all the joint components, and there is a destruction of articular cartilage, subchondral bone sclerosis and osteophyte formation, with varying degrees of inflammation [1-3]. The relationship between bone mineral density (BMD) and osteoarthritis has often been studied, with varied results. Several studies suggest that osteoporosis and osteoarthritis are accompanied by changes in bone mass in opposite directions, both locally and systemically [4-7]. These studies indicated that patients with osteoarthritis had a greater BMD than controls at multiple levels, yet that finding has not been confirmed universally. In other studies, it has been associated with osteoarthritis of the knee with an increased risk of vertebral fractures and non vertebra [8]. Alterations in gait and propensity to fall may play a role. Conversely, high BMD may favor arthrosis, perhaps by increasing the load to which articular cartilage is subjected in the presence of a denser subchondral bone [9]. However, this issue is also controversial. In fact, in some experimental models of osteoporosis, the development of arthrosis seems to accelerate [10,11]. To provide new information on this matter, we analyze the trabecular bone of the femoral head, comparing hip fracture sufferers with patients who present hip osteoarthritis.
Material and methods
Patients
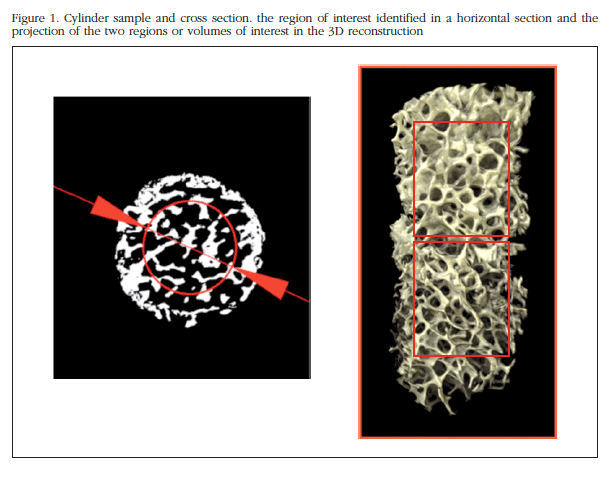
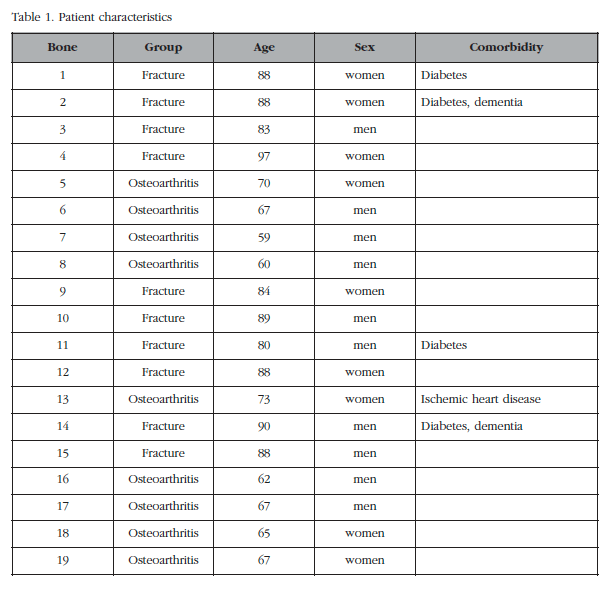
In all, 20 patients were included with severe coxarthrosis or hip fracture (femoral neck) in which it was necessary to place a prosthesis. We excluded those with those diseases that cause osteoporosis or secondary osteoarthritis (inflammatory diseases, advanced kidney failure, cancer, paralysis, treatment with corticosteroids or immunosuppressants, dysplasia, etc.) and fractures related to high-energy trauma. Trabecular bone cylinders of the femoral head were removed using a 6 mm diameter trocar needle (Figure 1) and the ends (3 mm adjacent to cortical bone and the fracture or surgical section) were also removed. The cylinders were obtained regardless of the state of the articular cartilage, or anatomical orientation. In one patient, we were unable to extract a good cylinder, so he was not included in the analysis. Consequently, the fracture group consisted of 10 patients (5 men and 5 women), aged 87±5 years. The arthrosis group was made up of 9 patients (5 men and 4 women) of 66±5 years (Table 1). The bone samples were collected within a research project aimed at determining the differential pathogenetic mechanisms in fractures and osteoarthritis, approved by the Cantabria Ethics Committee on Clinical Research. Patients gave informed written consent.
MicroCT Analysis
The cylinders were fixed in buffered 4% formaldehyde prior to analysis. The storage period in formaldehyde was 2-4 weeks for 12 samples, and 18-30 months in the rest (similar in both patient groups). After rinsing them with water, the study was conducted in a Microtrac 1172 Skyscan-Bruker, with 360o rotation, 75 kV voltage, 100 µA intensity, 16x16 mm field of view, 8-micron pixel size and an aluminum filter of 0.5 mm. Coronal sections (2,000x2,000 pixels) of all cylinders, with a separation between them of 1 pixel was reconstructed. NRecon SkyScan program was used for reconstruction. For calculations of structural parameter, the sections with uniform threshold were dichotomized and SkyScan CTanalyzer program was used. The global threshold values was defined taking into account all the analyzed samples. Each cut in a region of interest (ROI), centered in the sample, 4 mm in diameter (thus avoiding analysis of the periphery that may contain irregularities due to the extraction process). For tridimensional analysis, two regions or volumes of interest (VOI) consisting of two non-overlapping sets of consecutive sections, of equal size along the bone cylinder (Figure 1) defined in each sample. The sample ends and objects smaller than 100 voxels were excluded to avoid artifacts.
Statistical analysis
For each of the cylinders, the average value of the various parameters in the two regions studied (VOIs) as well as the variation among them was calculated. To analyze this variation for each parameter, the relative difference, as the resulting absolute value subtract 1 from the ratio of the value of each parameter in the two study regions (VOIs) was estimated. The Mann-Whitney test was used to analyze the differences between the average values of the two patient groups (fracture and osteoarthritis), as well as between men and women. The difference in standard deviations was analyzed by Levene's test.
Results
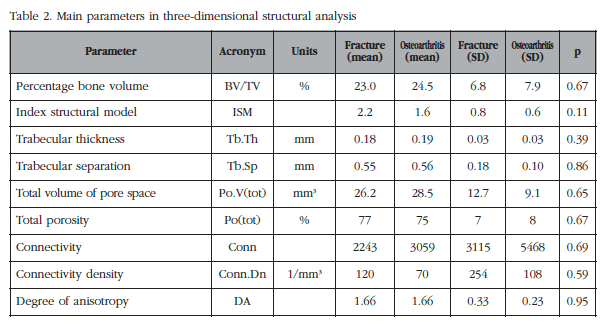
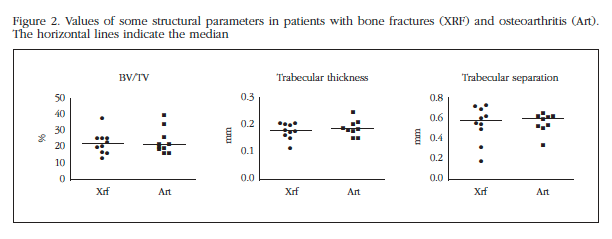
The average values of the main parameters analyzed are shown in table 2. In figure 2 the individual values of selected parameters are represented. As can be seen, no significant differences were found between fracture and osteoarthritis groups, or in terms of parameters (mean, median) in the central tendency, or as to the dispersion (variances).
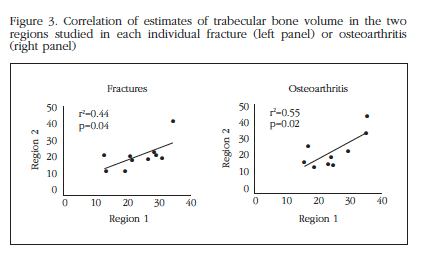
The differences between the two regions analyzed were also similar in both groups of patients (Figure 3). In fact, the average variation of the trabecular bone volume between the two regions was analyzed as 0.232 in the fractures and 0.236 in osteoarthritis (p=0.9).
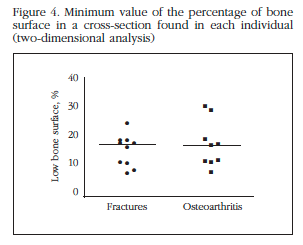
In each of the cylinders, 1,000 sections were scanned and corresponding to the percentage of bone surface (B.Ar/T.Ar), equivalent to trabecular bone volume in three-dimensional analysis dimensional parameter analyzed. The minimum value of the identified parameter found in all sections could be a better predictor of the maximum voltage that the bone can support before suffering a fracture [12,13]. As shown in figure 4, there were no differences between patient groups.
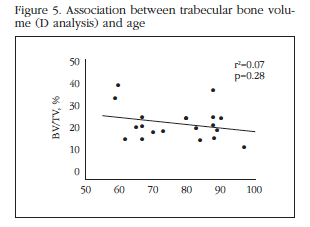
No significant association between age and bone volume (Figure 5), or crude analysis even taken into consideration when sex and group membership (fracture or osteoarthritis). Within each of the patient groups, we also found a significant Tb.Sp correlation and other microstructural parameters with age or sex. However, given the number of subjects included, this subgroup analysis had limited power.
Discussion
In this study we observed that the trabecular bone of the femoral head is similar in patients with hip fractures and in patients with advanced hip osteoarthritis in the volume of bone and different microstructural parameters. This result may not support the idea that opposite changes occur in these processes in bone mass, and specifically in the trabecular bone volume. We need to take into account that the subchondral bone was not included in the region to analyze, as it is located just below the articular surface, in which local changes occur in the form of sclerosis that are not representative of the overall skeletal situation [2,14].
In any case, the relationship between osteoporosis and osteoarthritis is a subject of debate. Several epidemiological studies have suggested that bone mass is increased in arthrosis [6,7,9,15], but others have not confirmed increased BMD [5,16]. In fact, some experimental studies suggest that osteoporosis may be a facilitator of the progression of some forms of arthrosis factor [10,17,18]. On the other hand, some studies have found an increased risk of fractures in patients with arthrosis [8], although it is difficult to determine the influence of a possible increased susceptibility to falls in individuals with advanced osteoarthritis. The reason for these differences is unclear. In part, it may be because osteoarthritis is not a homogeneous disorder, but there are epidemiological and pathogenic differences, not only in terms of the affected joints, but also in the type of alteration. In particular, there are relevant differences between "atrophic" and "hypertrophic" defined phenotypes depending on the absence or presence of osteophytes respectively. In fact, in a cohort analysis of Rotterdam, researchers observed that patients with atrophic type hip arthrosis had a higher risk of osteoporotic fractures [19].
There are also conflicting results regarding the local state of bone in patients with hip osteoarthritis and fractures. Thus, in some studies, increased bone mass (i.e., greater trabecular bone volume) has been identified in patients with coxartrosis [20,21], including a study of Montoya et al. Their design was similar to the present study, but samples were collected from the area of maximum load femoral head [22]. In some cases, the results may be influenced by a marked difference in age and sex distribution between groups [23], to exclude precisely the hip arthrosis group which had "osteoporosis" [20], having studied the femoral neck, instead of head, or for having studied a region very close to the subchondral plate [20]. Anyway, ours is not the only study which did not find any differences in trabecular bone between the two processes. Other authors published similar findings [24,25].
One limitation of our study is determined by variations in the orientation of the cylinders. This could theoretically increase the dispersion of results and therefore decrease the ability to find differences, as trabecular heads are not oriented uniformly in space. However, the absence of anisotropic differences of both groups suggests that this limitation may not play an important role. Likewise, the fact that two regions in each cylinder were analyzed independently and having obtained a similar correlation between them in both groups of patients, suggests that possible differences in the microstructure as a function of depth (after excluding the subchondral region) were not responsible for significant bias in the results. Another limitation of the study is that the cylinders were obtained at random, regardless of the state of cartilage. This could present a certain confusion, since it seems that the bone found beneath regions with damaged cartilage has a stronger trabecular structure than those located in areas under unharmed cartilage [26]. This may be the result of lower damping mechanical stresses in the areas lacking cartilage. The average age of patients in our two study groups was somewhat different, older in the fractured group. However, the age disparity highlighted even more the failure to find a lower bone volume in samples of patients with fracture. We excluded those fractures related to high-energy trauma (traffic accidents and falls from heights), so the hip fractures included could be considered due to fragility or osteoporosis. On the other hand, patients with osteoarthritis had no history of fragility fractures. We did not have a densitometry, so we could not completely exclude some degree of overlap between the groups.
Perhaps more surprising than the lack of differences between hip osteoarthritis and fractures is the absence of a relationship between the parameters analyzed and age, within the range studied. This is clearly contrary to the trend of BMD to decrease with age is generally observed in the skeleton. However, this result does not represent an isolated observation. In a study that included 37 individuals aged 40 to 90 years, Perilli et al. found no relationship between age and various structural parameters, including the volume of trabecular bone [13]. The reason for this peculiar behavior is unknown. It could be associated with biomechanical factors such as the persistent application of load on the femoral head when standing, with humoral factors dependent on the bone itself or other nearby tissues, including muscles, or the peculiar conditions of vascularization of the region. In any case, its clarification could improve our understanding of bone biology and perhaps open the way to new approaches for treating bone loss associated with aging.
Competing interests
The authors declare no conflict of interest in connection with this research study.
![]() Correspondence:
Correspondence:
José A. Riancho
Departamento de Medicina Interna
Hospital Universitario Marqués de Valdecilla
Universidad de Cantabria
Avda. Valdecilla, s/n
39008 Santander (España)
e-mail: rianchoj@unican.es
Date of receipt: 05/02/2016
Date of acceptance: 18/05/2016
Bibliography
1. Li G, Yin J, Gao J, Cheng TS, Pavlos NJ, Zhang C, et al. Subchondral bone in osteoarthritis: insight into risk factors and microstructural changes. Arthritis Res Ther. 2013;15(6):223. [ Links ]
2. Goldring SR. The role of bone in osteoarthritis pathogenesis. Rheum Dis Clin North Am. 2008;34:561-71. [ Links ]
3. Weinans H, Siebelt M, Agricola R, Botter SM, Piscaer TM, Waarsing JH. Pathophysiology of peri-articular bone changes in osteoarthritis. Bone. 2012;51(2):190-6. [ Links ]
4. Dequeker J, Aerssens J, Luyten FP. Osteoarthritis and osteoporosis: clinical and research evidence of inverse relationship. Aging Clin Exp Res. 2003;15:426-39. [ Links ]
5. Arokoski JP, Arokoski MH, Jurvelin JS, Helminen HJ, Niemitukia LH, Kroger H. Increased bone mineral content and bone size in the femoral neck of men with hip osteoarthritis. Ann Rheum Dis. 2002;61:145-50. [ Links ]
6. Chaganti RK, Parimi N, Lang T, Orwoll E, Stefanick ML, Nevitt M, et al. Bone mineral density and prevalent osteoarthritis of the hip in older men for the Osteoporotic Fractures in Men (MrOS) Study Group. Osteoporos Int. 2010;21:1307-16. [ Links ]
7. Hochberg MC, Lethbridge-Cejku M, Tobin JD. Bone mineral density and osteoarthritis: data from the Baltimore Longitudinal Study of Aging. Osteoarthritis Cartilage. 2004;12 Suppl A:S45-8. [ Links ]
8. Bergink AP, van der KM, Hofman A, Verhaar JA, van Leeuwen JP, Uitterlinden AG, et al. Osteoarthritis of the knee is associated with vertebral and nonvertebral fractures in the elderly: the Rotterdam Study. Arthritis Rheum. 2003;49(5):648-57. [ Links ]
9. Bergink AP, Uitterlinden AG, van Leeuwen JP, Hofman A, Verhaar JA, Pols HA, Bone mineral density and vertebral fracture history are associated with incident and progressive radiographic knee osteoarthritis in elderly men and women: the Rotterdam Study. Bone. 2005;37:446-56. [ Links ]
10. Bellido M, Lugo L, Roman-Blas JA, Castaneda S, Caeiro JR, Dapia S, et al. Subchondral bone microstructural damage by increased remodelling aggravates experimental osteoarthritis preceded by osteoporosis. Arthritis Res Ther. 2010;12(4):R152. [ Links ]
11. Herrero-Beaumont G, Roman-Blas JA, Largo R, Berenbaum F, Castaneda S. Bone mineral density and joint cartilage: four clinical settings of a complex relationship in osteoarthritis. Ann Rheum Dis. 2011;70(9):1523-5. [ Links ]
12. Perilli E, Baleani M, Ohman C, Fognani R, Baruffaldi F, Viceconti M. Dependence of mechanical compressive strength on local variations in microarchitecture in cancellous bone of proximal human femur. J Biomech. 2008;41(2):438-46. [ Links ]
13. Perilli E, Baleani M, Ohman C, Baruffaldi F, Viceconti M. Structural parameters and mechanical strength of cancellous bone in the femoral head in osteoarthritis do not depend on age. Bone. 2007;41(5):760-8. [ Links ]
14. Goldring MB, Goldring SR. Osteoarthritis. J Cell Physiol. 2007;213:626-34. [ Links ]
15. Hart DJ, Cronin C, Daniels M, Worthy T, Doyle DV, Spector TD. The relationship of bone density and fracture to incident and progressive radiographic osteoarthritis of the knee: the Chingford Study. Arthritis Rheum. 2002;46:92-9. [ Links ]
16. Lethbridge-Cejku M, Tobin JD, Scott WW Jr., Reichle R, Roy TA, Plato CC, et al. Axial and hip bone mineral density and radiographic changes of osteoarthritis of the knee: data from the Baltimore Longitudinal Study of Aging. J Rheumatol. 1996;23:1943-7. [ Links ]
17. Roman-Blas JA, Castaneda S, Largo R, Herrero-Beaumont G. Osteoarthritis associated with estrogen deficiency. Arthritis Res Ther. 2009;11:241. [ Links ]
18. Sandini L, Arokoski JP, Jurvelin JS, Kroger H. Increased bone mineral content but not bone mineral density in the hip in surgically treated knee and hip osteoarthritis. J Rheumatol. 2005;32:1951-7. [ Links ]
19. Castano-Betancourt MC, Rivadeneira F, Bierma-Zeinstra S, Kerkhof HJ, Hofman A, Uitterlinden AG, et al. Bone parameters across different types of hip osteoarthritis and their relationship to osteoporotic fracture risk. Arthritis Rheum. 2013;65(3):693-700. [ Links ]
20. Zhang ZM, Li ZC, Jiang LS, Jiang SD, Dai LY. Micro-CT and mechanical evaluation of subchondral trabecular bone structure between postmenopausal women with osteoarthritis and osteoporosis. Osteoporos Int. 2010;21(8):1383-90. [ Links ]
21. Tanck E, Bakker AD, Kregting S, Cornelissen B, Klein-Nulend J, Van Rietbergen B. Predictive value of femoral head heterogeneity for fracture risk. Bone. 2009;44(4):590-5. [ Links ]
22. Montoya MJ, Giner M, Miranda C, Vazquez MA, Caeiro JR, Guede D, et al. Microstructural trabecular bone from patients with osteoporotic hip fracture or osteoarthritis: its relationship with bone mineral density and bone remodelling markers. Maturitas. 2014; 79(3):299-305. [ Links ]
23. Marinovic M, Bazdulj E, Celic T, Cicvaric T, Bobinac D. Histomorphometric analysis of subchondral bone of the femoral head in osteoarthritis and osteoporosis. Coll Antropol. 2011;35(Suppl 2):19-23. [ Links ]
24. Fazzalari NL, Parkinson IH. Femoral trabecular bone of osteoarthritic and normal subjects in an age and sex matched group. Osteoarthritis Cartilage. 1998; 6(6):377-82. [ Links ]
25. Tsangari H, Kuliwaba JS, Fazzalari NL. Trabecular bone modeling and subcapital femoral fracture. J Musculoskelet Neuronal Interact. 2007;7(1):69-73. [ Links ]
26. Chappard C, Peyrin F, Bonnassie A, Lemineur G, Brunet-Imbault B, Lespessailles E, et al. Subchondral bone micro-architectural alterations in osteoarthritis: a synchrotron micro-computed tomography study. Osteoarthritis Cartilage. 2006;14(3):215-23. [ Links ]











 text in
text in