1. MLO-Y4 cells modulate human peripheral blood mononuclear cells migration
Aedo Martín D1, Orellana Gómez-Rico JA1, Buendía Montes I2, Areta Jiménez FJ1, Ardura JA2, Rodríguez de Gortázar A2
1 Hospital Universitario Central de la Defensa Gómez-Ulla. Servicio de Cirugía Ortopédica y Traumatología. Madrid; 2 Instituto de Medicina Molecular Aplicada (IMMA). Facultad de Medicina. Universidad San Pablo CEU. Madrid
Osteocytes, the most abundant cell type in bone, are buried in the mineralized bone matrix but regulate bone remodeling by altering osteoblast and osteoclast function. The aim of the present study was to evaluate the response of peripheral blood mononuclear cells from patients with osteoporotic and non-osteoporotic fracture, to the factors secreted by MLO-Y4 osteocytic cells under static or mechanical conditions. We obtained 12ml of peripheral blood of non-osteoporotic patients older than 18 years with traumatic fracture of long bones and osteoporotic patients with hip fracture. Patients in treatment for osteoporosis, with a secondary osteoporosis state or a tumoral process were excluded from the study. The population was recruited in the last year in the Hospital Central de la Defensa Gómez Ulla (Madrid, Spain). Eighteen valid samples were obtained. 14 (78%) presented osteoporotic fracture and 4 (22%) presented traumatic long bone fracture without osteoporosis. We obtained the peripheral blood mononuclear cells and we evaluated their capacity to migrate under the presence of conditioned media from osteocytic cells. MLO-Y4 cells were subjected or not (static control) to mechanical stimulation by fluid flow (FF, 10 dyn/cm2, 15 min). Cell medium was changed and cells were cultured for additional 18 h and the conditioned medium (CM) was then collected. The migration assays were performed in Costar transwell cell culture chamber inserts (Corning Costar Corporation, Cambridge, MA) with an 8 μm pore size. Briefly, 5×104 cells were placed on the upper chamber in 20% of MLO-Y4 CM. After 6 hours, cells on the underside of the Transwell were fixed, stained with Crystal Violet and counted. We observed that CM from static MLO-Y4 cells increased monocyte migration (20.93%). In contrast, CM from mechanically stimulated MLO-Y4 cells inhibited monocyte migration (53.98%). The CM from static condition cells favored the migration of monocytic cells (p<0.001) with a correlation coefficient of 88%. The linear correlation coefficient of the regression line was 92%. The CM from mechanically stimulated cells inhibited the migration (p<0.001) with a correlation coefficient of 79.2%. The linear correlation coefficient of the regression line was 58.8%. Our present data indicates that human monocytic cells can respond to factors secreted by MLO-Y4 cells modulating their migration.
2. Evolution of serum sclerostin six months after liver transplantation: different response compared with bone turnover markers
Allo G1, Librizzi S1, Aramendi M2, Jiménez C3, Hawkins F1, Martínez G1
1 Endocrinology Service; 2 Laboratory Service; 3 General Surgery Service. 12 de Octubre University Hospital. Madrid
Introduction: Increased risk of fracture and low bone mineral density are common findings in patients with liver transplantation. Bone markers, such as sclerostin (SCL), could have an important role in the increased risk of fracture because it acts as a negative regulator of bone formation. In spite of this, there are no studies about the evolution of bone markers after the procedure.
Aim: The aim of our study was to evaluate SCL and other bone markers in a cohort of patients with liver transplantation (LT).
Patients and methods: This is a prospective, single-center study. 42 patients with LT were enrolled. Serum sclerostin, b-cross laps (b-CTX) and osteocalcin (OC) were measured at baseline (24 hours before the transplantations) and 1, 3 and 6 months after the procedure. Data are expressed as mean±s.d. for continuous variables. Pearson’s correlation coefficient was employed to test correlations among bone turnover markers. Student’s t-test was used to compare bone markers levels (from baseline to 6 months). A p-value <0.05 was considered as statistically significant.
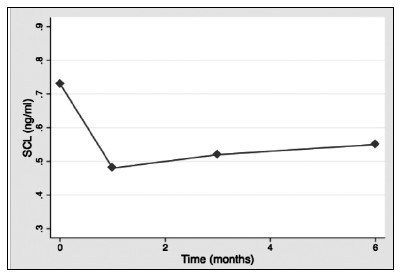
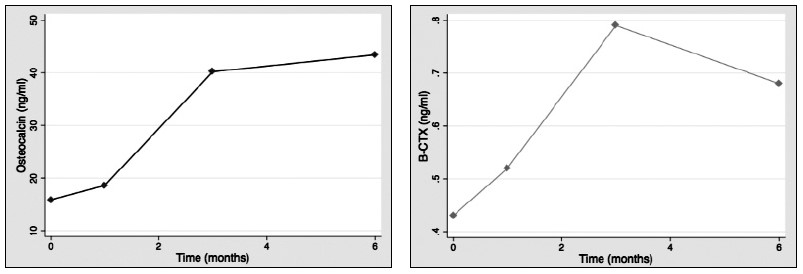
Results: 28:14 (male/female) patients were included. Mean age was 58.6±12.5 years. Bone markers levels at baseline were: SCL: 0.73±0.26 (ng/ml); b-CTX: 0,43±0.25 (ng/ml); OC 15.83±7.02 (ng/ml). No significant differences between patients with or without DM before transplantation. SCL levels decreased significantly during the study (1st month: 0.48±0.14 p<0.001; 3rd month 0.52±0.14, p=0.002; 6th month: 0.55±0.14, p=0.035)(Fig. 1).OC increased significantly after one month (18.6±14.4: p<0.001) and three months (40.2±36.2; p=0.01)(Fig. 2).There was also an increase after six months, but this was non-significant. b-CTX increased throughout the study and after three months there was a significant increase compared to baseline (0.79±0.57; p<0.001), which persisted after sin months (0,68±0.33; p=0.01)(Fig. 3). There were no significant correlations between bone markers during the study.
Conclusions: Serum SCL shows a consistent decrease after liver transplantation, that is detectable in the first month after transplantation. This pattern of change contrasts with other bone turnover markers, that increased significantly after transplantation. Higher perioperatively levels of SCL could play a role in the increased fracture risk of these patients.
3. Soluble factors of prostate cancer cells induce bone pre-metastasic niche changes in mice with induced prostate tumors
Ardura JA, Gutiérrez Rojas I, Álvarez Carrión L, Alonso V
IMMA-Universidad San Pablo CEU. Madrid
Metastases are the leading cause of death by cancer. Recent studies have described pro-metastatic changes in organs where metastases later develop. It has been suggested that formation of a pre-metastatic niche by cross-talk between the tumor and the target organ favors circulating tumor cell colonization. The aim of our study was to analyze the effects of primary prostate tumors on the bone environment before the settlemet and propagation of the metastases.
An in vivo pre-metastatic prostate cancer model based on the implantation of prostate adenocarcinoma TRAMP-C1 cells in immunocompetent C57BL/6 mice was used. One month after TRAMP-C1 cell implantation –a period of time sufficient for prostate tumor developing without bone metastases formation- histomorphometric parameters and bone markers were analyzed in femurs of mice with and without TRAMP-C1-induced prostate tumors. Also, we studied the effects of conditioned medium of murine TRAMP-C1 or human prostate tumor LNCaP cells on mouse osteocyte-like MLO-Y4 cells and human osteoblasts, respectively.
In vivo, primary prostate tumors showed osteomimetic features including increased Runx2, osterix, osteocalcin and RANK-L mRNA expression. Femurs of TRAMP-C1-induced tumor mice showed decreased trabecular thickness, trabecular separation, eroded surface and number of osteoclasts/bone surface and an increase in trabecular number compared to non-tumor control mice. Other histomorphometric parameters showed no significant changes. Associated to these changes, elevated immunolabeling of Runx2, osterix and sclerostin bone markers in femurs of TRAMP-C1-induced tumor mice compared to control mice were observed.
In vitro, Runx2 and osterix expression was induced in MLO-Y4 and human osteoblasts by conditioned medium of TRAMP-C1 and LNCaP cells, respectively.
These results suggest that secreted soluble factors of prostate tumorogenic cells induce changes in the bone environment before metastatic implantation.
4. Functional effects of the p.Asp188Tyr Mutation in the geranylgeranyl diphosphate synthase (GGPS1) gene associated with bisphosphonate-related atypical femoral fractures
Roca Ayats N1, Dunford JE2, PeiYing Ng3, García Giralt N4, Cozar M1, Quesada Gomez JM5, Nogués X4, Prieto Alhambra D2,6, Graham Russell R2, Grinberg D1, Díez Pérez A4, Baron R3, Balcells S1
1 Dept of Genetics. University of Barcelona and CIBERER; 2 University of Oxford. (Reino Unido); 3 Division of Bone and Mineral Research. Dept of Oral Medicine. Harvard School of Dental Medicine (Estados Unidos); 4 Hospital del Mar Institute of Medical Investigation and CIBERFES. Barcelona; 5 Instituto Maimónides de Investigación. Cordoba and CIBERFES; 6 GREMPAL and CIBERFES
Background: A novel mutation in the GGPS1gene was found in 3 sisters with atypical femoral fracture (AFF) after long-term bisphosphonates (N-BPs). This gene encodes the GGPPS protein, a key component of the mevalonate pathway. We have now performed functional studies to assess the impact of the mutation.
Methods: The cDNA for both wild type and Asp188Tyr GGPPS were cloned into a bacterial expression vector and the resulting his-tagged proteins were purified using Ni sepharose followed by gel filtration. Analysis of the oligomerisation of the GGPPS monomers was undertaken using a Sephadex S300 gel filtration column. Enzyme activity was assayed using both substrates, farnesyl pyrophosphate and C14-isopentenyl pyrophosphate (400KBq/uMol) at 20uM in buffer containing 100 mM HEPES pH7.5, 2mM MgCl2, 0.1% Tween 20. Reactions were stopped after 10 mins at 37C by adding acidified methanol. Products were extracted directly into water immiscible scintillation fluid and quantified by scintillation counting. The effect of shRNA-mediated GGPS1 depletion was also assessed in cells.
Results: Asp188 is an active site residue of GGPP synthase, involved in the binding of the substrate via a magnesium salt bridge. As predicted, disruption of this residue led to greatly reduced enzyme activity. In the recombinant enzyme the mutant had 5.7% of wildtype activity (0.72±0.09 cpm/ng/min for the wildtype, 0.04±0.013 cpm/ng/min for the mutant (n=3)). Gel filtration experiments showed the wildtype enzyme to have a MW in excess of 200 KDa suggesting that it is present as a hexamer in line with previous findings. The mutant enzyme consistently showed two peaks corresponding to the hexamer and the monomer (a peak around 38 kDa), suggesting the mutant destabilises the oligomerisation of the enzyme.
Furthermore, shRNA-mediated GGPS1 depletion (>80%) in RAW 264.7 macrophages yielded significantly higher osteoclast numbers after mCSF-RANKL stimulation when compared to the corresponding non-target shRNA control. However, despite having higher osteoclast formation rates, osteoclasts with reduced GGPS1 expression had lower resorption activity when cultured on the bone-mimicking substrate Osteo Assay surface plates.
Conclusions: GGPS1gene mutation greatly impairs the enzyme activity inducing biological effects that may contribute, likely with other factors notably BP exposure, to AFFs observed in patients carrying the mutation.
Abstract category: Genomics, Transcriptomics, Proteomics and Metabolomics of Musculoskeletal Disease