Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista de Osteoporosis y Metabolismo Mineral
versión On-line ISSN 2173-2345versión impresa ISSN 1889-836X
Rev Osteoporos Metab Miner vol.9 no.4 Madrid nov./dic. 2017
https://dx.doi.org/10.4321/s1889-836x2017000400004
Originals
Influence of vitamin D on biomechanical microstructure and properties of patients with hip fracture
1 Departamento de Medicina - Facultad Medicina - Universidad de Sevilla - Sevilla (España)
2 Unidad de Osteoporosis - Servicio Medicina Interna - Hospital Universitario Virgen Macarena - Sevilla (España)
3 Departamento de Citología Normal y Patológica - Facultad de Medicina - Universidad de Sevilla - Sevilla (España)
Introducción
Hip fracture is one of the main and most dreaded complications of osteoporotic disease. Among the risk factors that favor this type of fracture include a greater tendency to fall and a decrease in bone strength. Bone mineral density (BMD), the rate of bone remodeling, geometry, microstructure and bone tissue mineralization are fundamental properties associated with bone resistance 1.
Vitamin D is known to be essential, among other factors, in the maintenance of musculoskeletal health. Proper levels of 25-hydroxivitamin D (25(OH)D) are needed to maintain the homeostasis of calcium metabolism and an insufficiency of these leads to a lower intestinal absorption of calcium, decreased levels of serum calcium, increased secretion of PTH, excessive rate of bone remodeling and, therefore, a lower amount and bone quality 2. Furthermore, levels below 30 ng/mL have been found to be associated with significant defects of bone mineralization and increase in the osteoid substance 3. All of these disorders produce a decrease in bone strength and, thus, a greater risk of fracture. As for the muscle, several studies have shown the positive relationship between 25(OH)D levels and muscle strength, especially in the lower extremities in the elderly 4,5. Other research indicates muscular weakness and pain, as characteristic symptoms of Vitamin D-deficient syndromes 6, as well as increased muscle strength and balance and reduced risk of falls following the administration of adequate vitamin D supplements 7,8,9,10. Two meta-analyzes evaluating controlled, double-blind and randomized studies conclude the beneficial effect of vitamin D supplementation, with a 19% reduction in falls, an 18% risk of hip fracture and 20% of the risk of any type of non-vertebral fracture 11,12. These same studies pointed out that anti-fall and antifracture efficiency of 25(OH) D is achieved when their levels are above 24 and 30 ng/mL, respectively.
In the adult population, a high frequency of inadequate levels of vitamin D has been reported, below a variable threshold that ranges between 20-40 ng/mL of 25(OH)D, according to the different authors, in several studies carried out in multiple communities in Europe and the United States 13,14,15. In Spain, this has also been observed in the elderly population with different characteristics in different regions, with values below 15 ng/mL being described in 68% of 77-year-olds living at home, compared to 100% of those institutionalized in Andalusia 16, a somewhat lower frequency in Cantabria 17, and even lower levels (4.6 ng/mL as mean values) in patients with hip fracture in Madrid 18.
These data reveal, on the one hand, the importance of vitamin D on muscle health and skeletal integrity and, on the other hand, the frequency of insufficient levels of the hormone among the adult population in general. However, the impact of serum vitamin D levels on the microstructural and biomechanical characteristics of bone tissue in patients with hip fracture has not been reported to date. Therefore, the main objective of our study is to ascertain if vitamin D levels influence the microarchitecture and biomechanical properties of the bone tissue. Secondly, we also analyze the association of these levels with biochemical markers of bone remodeling and with hormones that influence bone formation and resorption (parathormone -PTH-, and insulin-like growth factor -IGF-I-, respectively) parameters, all of which contribute to bone quality and, therefore, the risk of osteoporotic fracture.
Material and methods
1. Patients
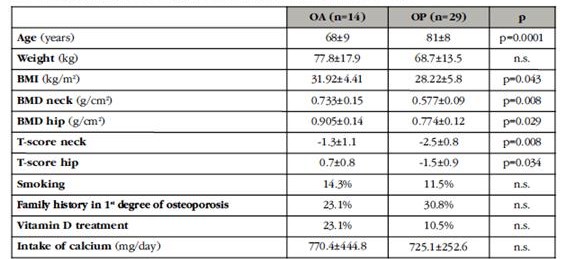
A cross-sectional observational study of 43 patients aged 50-93 years who underwent hip arthroplasty. The first group consisted of 29 (6 male and 23 female) patients with nontraumatic hip fracture (OP), considered as idiopathic (involutive) osteoporosis. The second, as a comparative group, consisted of 14 patients (6 men and 8 women) with osteoarthritis (OA) and values of bone mass, at the hip level, T-score >(-2.5), with no personal history of fracture Osteoporosis or disease with influence on bone metabolism. Normal renal function was required.
All patients were recruited from the Traumatology and Orthopedics Service during the period from June 2014 to June 2015. Blood samples for biochemical determinations were collected in pairs with cases and controls in order to avoid seasonal bias in results. The protocol was reviewed and approved by the Center's Ethics Committee and all patients gave their informed consent.
The following data were collected: age, weight, body mass index (BMI), toxic habits (alcohol intake and smoking), semi-quantitative calcium intake (1 glass of milk=200 mg/day, 1 milk derivative=200 mg/day, 1 serving of cheese=200 mg/day), first-degree family history with osteoporotic fracture, treatment with vitamin D supplements and/or antiresorptive drugs. Bone mass was evaluated in the contralateral hip in the period between 15-30 days after surgery.
Bone samples were collected from the extracted femoral head for microstructural analysis and study of bone biomechanical properties after compression test.
2. Biochemical parameters
To carry out the biochemical determinations, blood samples were taken from the patients, fasting, in the first 48 hours after surgery. The following parameters, related to bone metabolism, were analyzed: calcium corrected for protein levels, phosphorus, reabsorption bone remodeling markers (carboxyl-terminal telopeptide of type I or β-CTX collagen) and formation (amino terminal propeptide of procollagen type I or PINP, parathormone (PTH), 25-hydroxyvitamin D (25 (OH) D) and insulin-like growth factor (IGF-I).
Serum levels of corrected calcium and phosphorus were assessed using the DAX-96 autoanalyzer. The levels of β-CTX and PINP were analyzed by immunoassay (electro-chemiluminescence), with the autoanalyzer COBAS e 601 (Roche, Spain), with coefficients of variation (CV) being interassayed <7.6% and <4.2%, respectively. Serum PTH was measured by immunoassay (electro-chemiluminescence), with autoantibody ADVIA Centaur (Siemens, Germany), with the CV interassay <5.8%. Serum 25(OH)D was analyzed by direct competitive immunoassay (electro-chemiluminescence), with the autoanalyzer LIAISON (DiaSorin, Italy), with the interassay CV <5.5%. Finally, serum IGF-I was quantified by immunoassay (electro-chemiluminescence), with the autoanalyzer IMMULITE (Siemens, Germany), with CV interassay being <3.9%.
3. Bone mass evaluation
Bone mineral density of hip and contralateral femoral neck was quantified using dual X-ray densitometry (DXA), with a Hologic-Densitometer (Hologic Inc.). The CV in vivo was 1% (total BMD).
4. Microstructural and biomechanical study of bone tissue
The analysis of the microstructure and the biomechanical properties has been made from the bone samples taken at the time of the surgical intervention for the prosthesis collation. From each femoral head, a trabecular bone cylinder from the primary compression region has been extracted with the longitudinal axis of the cylinder aligned with the main trabecular direction (MTD) (Figure 1).

Figure 1 Localization of the trabecular bone cylinder extraction for the analysis of the microstructure and biomechanical properties
Microstructural analysis of the biopsies has been carried out using computerized microtomography (micro-CT) using Bruker SkyScan 1172. The scanning of the sample was carried out at a resolution of 11 μm, taking 2 images for each step of sample rotation (0.40°/pass, 180° total rotation). The images obtained have been reconstructed using the modified Feldkamp algorithm and later used for the quantitative and qualitative analysis of the trabecular bone microstructure.
The quantitative variables were: bone volume fraction (BV/TV), trabecular thickness (Tb.Th), trabecular number (Tb.N), trabecular separation (Tb.Sp), degree of anisotropy (DA), which is a measurement of the symmetry of the object, or the presence/absence of structures aligned in a given direction, model structure index (MSI), indicating the relative prevalence of trabeculae in the form of plates or in the form of tube-cylinder and connectivity (trabecular pattern factor, Tb.Pf), which is an index of inverse connectivity, so that the higher the value the less connected the trabeculae are.
To assess biomechanical properties, uniaxial compression tests were carried out using the IGFA (image-guided failure analysis), applying a maximum force of 200 N, without reaching the elastic resistance limit of the sample. The variables that were quantified to describe the mechanical behavior of the bone tissue were the maximum stress (σ), or maximum internal resistance of the object to a force acting on it. The maximum deformation (ε), which represents the changes in the dimensions of the object subjected to the action of force, from which microfractures occur and Young’s modulus, or elastic modulus, which represents the slope of the elastic region.
5. Statistical analysis
The IBM SPSS statistical package version 20.0 (USA) was used. For the statistical analysis of the results of quantitative variables, we carried out comparisons of Student's t-means for independent samples. We did univariate linear analysis (ANCOVA) to take into account possible confounding factors such as age and BMI. Pearson's correlation test was used to assess the association between variables. To study the qualitative variables, we have analyzed contingency tables, χ2. In all cases, a level of p <0.05 was required to consider significant differences.
Results
The characteristics of the patients studied in each of the groups are shown in table 1. As expected, patients with hip fracture had a significantly higher age, 81±8 vs 68±9 years, p=0.001, a lower BMI 28.2±5.8 vs 31.9±4.4, p=0.043 and lower hip mass values (p<0.05 for all locations). Since the group of patients with fracture and osteoarthrosis were different in terms of age and BMI, the parameters analyzed were adjusted for these variables. In terms of habits, family history of first-degree osteoporosis, treatment with antiresorptive drugs and supplementation with vitamin D and calcium were similar in both groups.
Table 1 Characteristics of the study population. Values expressed as mean ± SD
n.s.: not significant.
1. Serum values of parameters related to bone metabolism
The biochemical parameters of the patients studied are shown in table 2. Serum levels of 25(OH)D were significantly lower in patients with fracture than in the group of patients with osteoarthritis (10.9±6.9 vs 18.1±10.7 ng/mL, p=0.02). The values of PTH (60±41.1 vs 38.2±17.3 pg/mL, p=0.029) and the bone resorption marker β-CTX (0.61±0.26 vs 0.36±0.19 ng/mL, p=0.04) were significantly higher in the first group. Levels of corrected serum calcium, phosphorus, bone formation marker PINP and IGF-I were comparable in both groups. After adjustment for age and BMI, we verified that the differences that we found for 25(OH)D and β-CTX remained significant. The adjusted values for 25(OH)D were 10.7±(95% CI 6.6-14.7) ng/mL for the hip fracture group and 19.6±95% CI, 7-25.5) ng/mL, for that of arthrosic patients, (p=0.027). The levels of β-CTX adjusted for the same variables were 0.63±(95% CI 0.51-0.7) ng/mL, in the group of patients with hip fracture and 0.30±95% 0.1-0.5) ng/ml in the arthrosis group (p=0.012).
Table 2 Serum values of biochemical parameters of the study population. Values expressed as mean ± SD

n.s.: not significant.
Levels of 25(OH)D showed a significant and positive correlation with serum IGF-I (r=0.338, p=0.044) and negative with β-CTX levels (r=-0.483, p=0.003). PTH levels were significantly negatively correlated with BMD-hip (r=-0.617, p=0.005) and positively with trabeculae separation (r=0.530, p=0.006).
2. Bone tissue microstructure and biomechanics
Microstructural indices indicate lower bone quality in the group of patients with hip fracture than the arthrosic group. In figure 2, we show the result of scanned images of two patients, each belonging to a study group.

Figure 2 Reconstruction of scanned images of patient biopsies with osteoarthritis (A) and hip fracture (B)
Although no parameter presented a statistically significant difference, we verified that the values of percentage of bone volume, as well as the thickness and number of trabeculae were lower in patients with fracture, whereas the separation between them was greater (Table 3).
Table 3 Microstructure and biomechanical bone properties of the study group. Values expressed as mean ± SD

BV/TV: bone volume fraction; Tb.th: trabecular thickness; Tb.Sp: trabecular separation; Tb.N: number of trabeculae; Tb.Pf: trabecular connectivity; SMI: structural model index; AD: degree of anisotropy; σ: maximum voltage; ε: maximum deformation.
The trabecular pattern index (inverse index of connectivity) presented higher values in the group of fractured patients. The structure index of the model, which implies a relative prevalence of trabeculae in the form of tube-cylinder compared to those of plate form, was higher in the group of patients with hip fracture, indicating a greater number of trabeculae in the form of a tube-cylinder, less resistant in these patients.
Biomechanics studies showed that Young's modulus values, maximal strain and maximum deformation, after applying compression tests, were lower in patients with hip fracture than in patients with osteoarthritis, although these differences were not significant (Table 3).
3. BMD and bone structure results in patients with insufficient levels of 25(OH)D (<20 ng/mL)
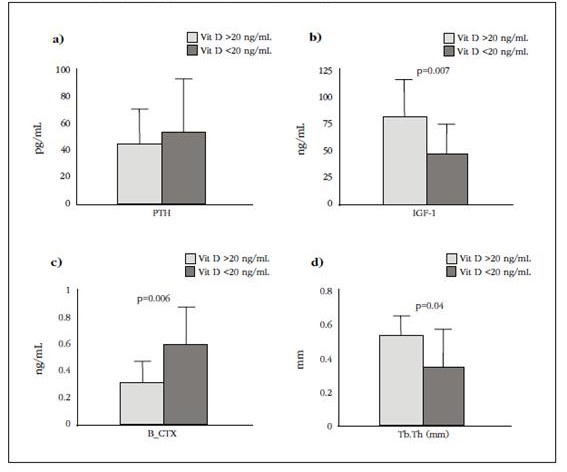
Serum levels of 25(OH)D in the studied population have shown very low values, in the range of 4.0-41.6 ng/mL when dividing patients into two groups, considering that these levels were less than or equal to 20 ng/mL or higher, we found the following results: the two populations were similar in age, weight, BMI, bone mass values and biomechanical characteristics of bone tissue. However, those with levels below 20 ng/mL had higher serum β-CTX values (0.58±0.25 vs 0.30±0.15 ng/mL, p=0.006), lower IGF-I (49.8±27.0 vs 83.5±35.6 ng/mL, p=0.007) and the bone structure showed a smaller trabecular width (0.34±0.17 vs 0.50±0.1 mm, p=0.04) (Figure 3).
Discussion
This study allowed us to compare serum levels of vitamin D in patients with and without osteoporotic hip fracture and the association of these levels with markers of bone remodeling, hormones regulating bone metabolism, BMD, microstructural indexes and biomechanical properties of the femoral neck in these patients.
We found that patients with osteoporotic hip fracture had significantly lower serum 25(OH)D levels than non-fractured patients. In addition, we noted a high prevalence in elderly people with deficient levels (<20 ng / mL), especially in the OP group, in line with what was observed by other authors 13,14,15,16,17,18, despite having approximately 3,000 hours of sunshine per year in our environment. As is known, insufficient levels of vitamin D are associated with an increased risk of falls and osteoporotic fractures 11,12. In our study, we also verified that patients with osteoporotic hip fracture also present higher serum levels of β-CTX and PTH, together with lower BMD values in all hip-measured locations, compared to patients without fracture. In addition, we demonstrated a significant negative correlation between 25(OH)D and β-CTX levels, as well as between PTH and BMD levels.
Taken together, these data once again explain that low levels of 25(OH)D are associated with high levels of PTH, that inducing a greater bone turnover would explain the higher values of bone remodeling markers. The imbalance in the levels of these parameters causes trabecular deterioration that could lead to increased risk of fracture. A high rate of bone remodeling increases the number of resorption cavities. These cavities act as "areas of stress accumulation", foci of weakness that may increase the risk of microfractures and macrofractures 19. Excessive resorption may also lead to perforation of the trabeculae and permanent loss of connectivity. All these alterations described at the microstructural level of the trabecular bone make it less resistant to the load and, therefore, with an increased risk of fracture 20. In our study, all individuals with non-traumatic hip fracture and with osteoarthritis, except one in the latter group, had levels of 25(OH)D <40 ng/mL. Some authors report that men with radiological osteoarthritis of the hip have lower levels of 25(OH)D and a prevalence of deficiency higher than the control population 21. It is generally known that up to this level of 40 ng/mL an inverse relationship between vitamin D and PTH 16 is described. Although a low calcium intake may also be responsible for increasing PTH levels, in our case we found that calcium intake was low but similar in both study groups (<800 mg/day). Although this is a factor to be taken into account, we know that insufficient levels of vitamin D lead to elevated levels of PTH, despite adequate calcium intake, and conversely, sufficient levels of vitamin D maintain normal levels of PTH despite calcium intake <800 mg/day 22.
Although patients with hip fracture presented lower BMD values than patients with osteoarthritis at all hip-measured locations, we did not find an association between these values and serum levels of 25(OH)D, as reported by other authors 23. However, we would like to point out that when all patients studied according to their 25(OH)D levels were <20 ng/mL or higher, the group of patients with lower levels also had lower levels of hip BMD, of 0.06 gHA/cm2. These data may not be important at the individual level, but in terms of population level, taking into account each decrease of 1 standard deviation (SD), the risk of hip fracture increases by an average of 2.6 times 24,25. This would imply that, with the lowest bone mass found, the risk of fracture may be increased up to 1.5 times.
Many experts, based on broad population studies, have jointly pointed out that minimum levels of 25(OH)D of at least 20-30 are desirable to maintain overall health integrity, and bone in particular, Ng/mL (50-75 nM) 26,27,28,29. The negative effect of insufficient levels of vitamin D on bone has been assessed by surrogate factors such as PTH, BMD, and markers of bone remodeling. It has also been directly analyzed through its association with bone properties involved in bone strength and, therefore, the risk of fractures. In this sense, it has been verified by histo-morphometric studies of iliac crest that vitamin D insufficiency is associated with mineralization defects, showing higher levels of surface and volume of osteoid, and concluding that hormone levels higher than 30 Ng/mL to prevent pathological accumulation of osteoid 3. One of the fundamental aspects of our study is the assessment of microarchitecture and biomechanical properties of the femoral neck of patients with and without osteoporotic hip fracture. Although the differences in the different parameters were not significant in any of the cases, as has been pointed out by other authors 30,31, we found that patients with hip fracture presented a percentage of bone volume (BV/TV), a number of trabeculae (Tb.N) and a width of these (Tb.th) 16%, 15% and 13% lower, respectively, than those of patients with osteoarthritis. In addition, the trabeculae were less connected and predominant than those presented as cylinder-tube in the fractured group, which is related to a lower bone resistance 32. Along with this, biomechanical parameters also showed results between 10-14% lower in patients with hip fracture. We would like to highlight, as the most important point of this study, that it analyzes for the first time the serum levels of vitamin D, directly related to the microstructural properties of bone tissue in people with hip fracture. When comparing hormone levels among patients, we found that levels below 20 ng/mL were associated with a significant reduction in trabecular width, in addition to higher levels of β-CTX, indicating a greater activity of bone resorption, together with lower levels of IGF-I. Vitamin D status reportedly contributes to the determination of serum IGF-I levels 33. In turn, IGF-I has been shown to stimulate renal 1α-hydroxylase activity, contributing to phospho-calcium metabolism, in addition to benefitting bone mass 34, associated with its reduction to a decrease in bone formation that contributes to bone loss in senile osteoporosis 35.
Our study has several limitations, especially in terms of sample size, which is relatively small. Furthermore, the dispersion of results of structural and mechanical parameters could be remedied, at least in part, with larger studies.
Overall, given the results obtained, we may conclude that the elderly population has a deficient vitamin D status, that these levels are even lower among patients with osteoporotic hip fracture and that serum concentrations below 20 ng/mL of the hormone can, directly or indirectly through PTH and IGF-I, condition an alteration in bone remodeling (β-CTX elevation) and BMD, with a consequent repercussion at the microstructural level (Tb.Th, among others) that lead to it being a low resistance bone where fractures easily occur.
Conclusions
These results indicate that patients with hip fracture have lower levels of 25(OH)D than patients with osteoarthritis, and that these induce raised PTH and increased bone resorption (with consequent increase in β-CTX levels) leading to decreased bone mass, decreased quality and increased risk of fracture. These alterations are more pronounced in patients with serum levels of 25(OH)D <20 ng/mL, in which there is also a decrease in IGF-I and trabecular width at the structural level of the femoral neck trabecular bone.
Acknowledgements:
Our sincere thanks to all the participants in the study and to the staff of the Unit of Management of Orthopedics and Traumatology of the Macarena University Hospital Orthopedics and Traumatology Department (Spain)
REFERENCES
1. Veldurthy V, Wei R, Oz L, Dhawan P, Jeon YH, Christakos S. Vitamin D, calcium homeostasis and aging. Bone Res. (Internet). 2016;4:16041. [ Links ]
2. Sosa Henríquez M, Gómez de Tejada Romero MJ, Recker RR, Cannata Andía JB, Del Pino Montes J, Díaz Curiel M, et al. Papel del calcio y la vitamina D en el tratamiento de la osteoporosis. Rev Osteoporos Metab Miner. 2010;2(1):61-72. [ Links ]
3. Priemel M, von Domarus C, Klatte TO, Kessler S, Schlie J, Meier S, et al. Bone mineralization defects and vitamin D deficiency: histomorphometric analysis of iliac crest bone biopsies and circulating 25-hydroxyvitamin D in 675 patients. J Bone Miner Res. 2010;25(2):305-12. [ Links ]
4. Wicherts IS, van Schoor NM, Boeke a JP, Visser M, Deeg DJH, Smit J, et al. Vitamin D status predicts physical performance and its decline in older persons. J Clin Endocrinol Metab. 2007;92(6):2058-65. [ Links ]
5. Bischoff-Ferrari HA, Dietrich T, Orav EJ, Hu FB, Zhang Y, Karlson EW, et al. Higher 25-hydroxyvitamin D concentrations are associated with better lower-extremity function in both active and inactive persons aged 60 y. Am J Clin Nutr. 2004;80(3):752-8. [ Links ]
6. Schott GD, Wills MR. Muscle weakness in osteomalacia. Lancet. 1976;307(7960):626-9. [ Links ]
7. Annweiler C, Beauchet O. Questioning vitamin D status of elderly fallers and nonfallers: a meta-analysis to address a 'forgotten step'. J Intern Med. 2015;277(1):16-44. [ Links ]
8. Pfeifer M, Begerow B, Minne HW, Suppan K, Fahrleitner-Pammer A, Dobnig H. Effects of a long-term vitamin D and calcium supplementation on falls and parameters of muscle function in community-dwelling older individuals. Osteoporos Int. 2009;20(2):315-22. [ Links ]
9. Pfeifer M, Begerow B, Minne HW, Abrams C, Nachtigall D HC. Effects of a short-term vitamin D and calcium supplementation on body sway and secondary hyperparathyroidism in elderly women. J Bone Min Res. 2000;15(6):1113-8. [ Links ]
10. Broe KE, Chen TC, Weinberg J, Bischoff-Ferrari HA, Holick MF KD. A higher dose of vitamin d reduces the risk of falls in nursing home residents: a randomized, multiple-dose study. J Am Geriatr Soc. 2007;55(2):234-9. [ Links ]
11. Bischoff-Ferrari HA, Dawson-Hughes B, Staehelin HB, Orav JE, Stuck AE, Theiler R, et al. Fall prevention with supplemental and active forms of vitamin D: a meta-analysis of randomised controlled trials. BMJ. 2009;339:b3692. [ Links ]
12. Bischoff-Ferrari HA, Willett WC, Wong JB, Stuck AE, Staehelin HB, Orav EJ, et al. Prevention of nonvertebral fractures with oral vitamin D and dose dependency: a meta-analysis of randomized controlled trials. Arch Intern Med. 2009;169(6):551-61. [ Links ]
13. Kuchuk NO, van Schoor NM, Pluijm SM, Chines A, Lips P. Vitamin D status, parathyroid function, bone turnover, and BMD in postmenopausal women with osteoporosis: global perspective. J Bone Miner Res. 2009;24(4):693-701. [ Links ]
14. Bandeira F, Griz L, Dreyer P, Eufrazino C, Bandeira C, Freese E. Vitamin D deficiency: A global perspective. Arq Bras Endocrinol Metab. 2006;50(4):640-6. [ Links ]
15. Holick MF. The vitamin D epidemic and its health consequences. J Nutr. 2005;135(11):2739S-48S. [ Links ]
16. Quesada JM, Jans I, Benito P, Jimenez JA, Bouillon R. Vitamin D status of elderly people in spain. Age Ageing. 1989;18(6):392-7. [ Links ]
17. Castillo Suárez M, Sosa Henríquez M. Modificación de las hormonas calciotropas y los marcadores bioquímicos de remodelamiento óseo, en función de la edad y el sexo, en una población anciana institucionalizada. Rev Esp Geriatr Gerontol. 1998;33:349-56. [ Links ]
18. Alarcón T, González-Montalvo JI, Hoyos R, Diez-Sebastián J, Otero A, Mauleon JL. Parathyroid hormone response to two levels of Vitamin D deficiency is associated with high risk of medical problems during hospitalization in patients with hip fracture. J Endocrinol Invest. 2015;38(10):1129-35. [ Links ]
19. Geissler JR, Bajaj D, Fritton JC. American Society of Biomechanics Journal of Biomechanics Award 2013: cortical bone tissue mechanical quality and biological mechanisms possibly underlying atypical fractures. J Biomech. 2015;48(6):883-94. [ Links ]
20. van der Linden JC, Weinans H. Effects of microarchitecture on bone strength. Curr Osteoporos Rep. 2007;5(2):56-61. [ Links ]
21. Chaganti RK, Parimi N, Cawthon P, Dam TL, Nevitt MC, Lane NE. Association of 25-hydroxyvitamin D with prevalent osteoarthritis of the hip in elderly men: The osteoporotic fractures in men study. Arthritis Rheum. 2010;62(2):511-4. [ Links ]
22. Steingrimsdottir L, Gunnarsson O, Indridason OS, Franzson L, Sigurdsson G. Relationship between serum parathyroid hormone levels, vitamin D sufficiency, and calcium intake. JAMA. 2005;294(18):2336-41. [ Links ]
23. Bischoff-Ferrari HA, Dietrich T, Orav EJ, Dawson-Hughes B. Positive association between 25-hydroxy vitamin D levels and bone mineral density: a population-based study of younger and older adults. Am J Med. 2004;116(9):634-9. [ Links ]
24. Cummings SR, Browner W, Cummings SR, Black DM, Nevitt MC, Browner W, et al. Bone density at various sites for prediction of hip fractures. Lancet. 1993;341(8837):72-5. [ Links ]
25. Ensrud KE, Taylor BC, Paudel ML, Cauley JA, Cawthon PM, Cummings SR, et al. Serum 25-hydroxyvitamin D levels and rate of hip bone loss in older men. J Clin Endocrinol Metab. 2009;94(8):2773-80. [ Links ]
26. Dawson-Hughes B, Mithal A, Bonjour JP, Boonen S, Burckhardt P, Fuleihan GEH, et al. IOF position statement: Vitamin D recommendations for older adults. Osteoporos Int. 2010;21(7):1151-4. [ Links ]
27. Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911-30. [ Links ]
28. Gómez de Tejada Romero MJ, Sosa Henríquez M, Del Pino Montes J, Jódar Gimeno E, Quesada Gómez JM, Cancelo Hidalgo MJ, et al. Documento de posición sobre las necesidades y niveles óptimos de vitamina D. Rev Osteoporos Metab Miner. 2011;3(1):53-64. [ Links ]
29. Atkinson SA. The new dietary reference intakes from the Institute of Medicine for calcium and vitamin D. Perspect Infirm. 2011;8(5):5. [ Links ]
30. Blain H, Chavassieux P, Portero-Muzy N, Bonnel F, Canovas F, Chammas M, et al. Cortical and trabecular bone distribution in the femoral neck in osteoporosis and osteoarthritis. Bone. 2008;43(5):862-8. [ Links ]
31. Zhang ZM, Li ZC, Jiang LS, Jiang SD DL. Micro-CT and mechanical evaluation of subchondral trabecular bone structure between postmenopausal women with osteoarthritis and osteoporosis. Osteoporos Int. 2010;21(8):1383-90. [ Links ]
32. Cohen A, Liu XS, Stein EM, McMahon DJ, Rogers HF, LeMaster J, et al. Bone microarchitecture and stiffness in premenopausal women with idiopathic osteoporosis. J Clin Endocrinol Metab. 2009;94(11):4351-60. [ Links ]
33. Bogazzi F, Rossi G, Lombardi M, Tomisti L, Sardella C, Manetti L, et al. Vitamin D status may contribute to serum insulin-like growth factor I concentrations in healthy subjects. J Endocrinol Invest. 2010;34(8):200-3. [ Links ]
34. Bex M, Bouillon R. Growth hormone and bone health. Horm Res. 2003;60 Suppl 3:80-6. [ Links ]
35. Riggs BL. Endocrine causes of age-related bone loss and osteoporosis. Novartis Found Symp. 2002;242:244-7. [ Links ]
Ethics: The study was approved by the Institutional Ethics Committee (Macarena University Hospital, Seville, Spain) and informed consent was obtained from each participant.
Received: March 31, 2017; Accepted: May 21, 2017











 texto en
texto en