1. 3D analysis of the cortical and trabecular bone of elite female athletes involved in high- and low-impact sports
Humbert L1, Río L del5, Lizarraga A3, Bellver M4, Winzenrieth R1, Amani A5, Drobnic F34
1 Galgo Medical. Barcelona. Spain; 3 Football Club Barcelona. Spain; 4 Centro de alto Rendimiento. Sant Cugat del Valles. Barcelona. Spain; 5 CETIR Centre Medic. Barcelona. Spain
Purpose: To assess and compare the cortical and trabecular bone of the proximal femur of athletes involved in high-impact sports (football and volleyball) and low-impact sports (swimming, synchronised swimming and water polo) using DXA-derived 3D analysis.
Methods: Elite female athletes from the football first team of FC Barcelona and from the Spanish national team of volleyball, swimming, synchronised swimming and water polo were included in this study. Hip DXA scans were performed at the Medical Services of FC Barcelona (Barcelona, Spain) using a Lunar iDXA scanner (GE Healthcare, Madison, WI). Areal BMD (aBMD) were calculated at total femur. The 3D-SHAPER software (v2.7, Galgo Medical, Barcelona, Spain) was used to provide 3D analysis of the cortical and trabecular bone from hip DXA scans. Trabecular volumetric BMD (vBMD) and cortical surface BMD (sBMD) were calculated at total femur. DXA and DXA-derived 3D measurements calculated for every groups were compared using Student’s t-test.
Results: The number and mean age (±SD) of the female athletes involved in this study was N=60, 22±4 years (football); N=26, 23±6 years (volleyball); N=18, 19±4 years (swimming); N=25, 21±5 years (synchronised swimming); and N=14, 24±4 years (water polo). No difference in aBMD, trabecular vBMD and cortical sBMD was found between athletes involved in high-impact sports (football and volleyball). Among the groups involved in low-impact sports, water polo athletes had 10% higher aBMD, trabecular vBMD and cortical sBMD (p<0.05 for all measurements), compared to swimmers. They showed 7% higher cortical sBMD (p<0.05), compared to synchronised swimmers, while no significant differences were found for aBMD and trabecular vBMD. Athletes involved in high-impact sports had higher aBMD (12% to 21%), trabecular vBMD (17% to 34%) and cortical sBMD (11% to 27%), compared to low-impact sports. Distribution of the mean differences in cortical sBMD between football and swimming athletes are shown in Figure.

Figure Anatomical distribution of mean differences in cortical sBMD between football athletes and swimming athletes.
Conclusions: Athletes involved in high-impact sports exhibited higher densities in both cortical and trabecular compartments, compared to low-impact sports. Interestingly, water polo athletes have higher cortical density compared with other swimming athletes which could be explained by higher workout.
2. Is serum free DNA methylation a bone biomarker?
Real A del1, Sañudo C1, Garcia-Ibarbia C1, Valero MC1, Fraga MF4,5, Fernandez AF4,5, Perez-Campo FM3, Perez-Nuñez MI2, Laguna E2, Riancho JA1
1 Department of Internal Medicine. Hospital Universitario Marqués de Valdecilla-IDIVAL, University of Cantabria. Santander. Spain; 2 Department of Traumatology. Hospital U M Valdecilla. University of Cantabria. Santander. Spain; 3 Department of Molecular Biology. University of Cantabria-IDIVAL. Santander. Spain; 4 Nanomaterials & Nanotechnology Research Center (CINN-CSIC). University of Oviedo. Oviedo. Spain; 5 Cancer Epigenetics Laboratory. Institute of Oncology of Asturias (IUOPA). HUCA. University of Oviedo. Oviedo. Spain
Cell-free DNA (cfDNA) is present in fluids, such as urine and serum. It is an appealing molecular biomarker because it is easy to obtain without using invasive procedures. DNA methylation regulates gene expression and has specific profiles according to the tissue of origin. We have previously shown that methylation of SOST (gene encoding sclerostin) contributes to the regulation of sclerostin synthesis. In fact, there is an inverse correlation between SOST methylation and expression (Delgado-Calle et al., JBMR 2012). The aim of this study was to determine the methylation of the SOST promoter in cfDNA, in comparison with the methylation pattern in DNA from blood and bone cells.
For this study, 30 patients with osteoporotic hip fractures undergoing replacement surgery were included. From each patient, bone tissue, blood and serum samples were obtained. A second group of 28 osteoporotic patients was also included. Serum samples were obtained at baseline and after 6-months therapy with alendronate, teriparatide or denosumab. DNA was analysed by pyrosequencing that allowed the interrogation of 3 CpGs of the SOST promoter. Sclerostin levels in serum were measured with ELISA.
The methylation level of the sclerostin promoter was very similar in serum cfDNA (84±11%) and bone-derived DNA (86±3%), but lower than in blood cells DNA (94±3%). Pairwise comparisons revealed statistically significant differences between blood and serum (p=0.0001), and between blood and bone (p=0.007). However, there were no difference between serum and bone (p=0.21). Moreover, there was a positive correlation between DNA methylation in serum and DNA methylation in bone (r=0.56; p=0.000002). We did not find differences in sclerostin serum levels nor in cfDNA methylation before and after anti-osteoporosis therapy.
In conclusion, methylation of the SOST promoter in serum cfDNA is lower than in blood cell DNA and similar to bone DNA, suggesting that serum cfDNA might originate in bone cells. However, since we did not find significant changes in either serum sclerostin or SOST methylation after therapy with bone-active drugs, the role of cfDNA as a bone biomarker cannot be confirmed yet.
3. FGF23 impairs osteocyte maturation by inhibition of Wnt/b-catenin pathway and is associated with bone alterations in early CKD
Díaz-Tocados JM1,2,3,4, Rodríguez-Ortíz ME½3,4, Almadén Y1,5,6, Martínez-Moreno JM1,2,3,4, Herencia C½3,4, Vergara N½3,4, Carvalho C7,8,9, Frazão JM7,8,10, Rodríguez M½3,4, Muñoz-Castañeda JR½3,4
1 Maimonides Institute for Biomedical Research (IMIBIC). Cordoba. Spain; 2 Nephrology Service. Reina Sofia University Hospital Cordoba. Spain; 3 University of Cordoba. Spain; 4 Spanish Renal Research Network (REDinREN). Institute of Health Carlos III. Madrid. Spain; 5 Internal Medicine Service. Reina Sofia University Hospital. Cordoba. Spain; 6 Spanish Biomedical Research Networking Centre consortium for the area of Physiopathobgy of Obesity and Nutrition (CIBEROBN). Institute of Health Carlos III. Madrid. Spain; 7 Braga Hospital. Department of Nephrology. Portugal; 8 Institute of Investigation and Innovation in Health (I3S). University of Porto. Portugal; 9 National Institute of Biomedical Engineer (INEB). University of Porto. Portugal; 110 Department of Nephrology. São João Hospital Center. Porto. Portugal
Patients with chronic kidney disease patients is associated with reduction of bone mineral density and fractures. In these patients Fibroblast Growth Factor 23 (FGF23) is markedly increased. The direct effects of FGF23 on bone cells is not clear. The effects of high FGF23 in bone were studied in vivo in an experimental model of heminephrectomized rats (1/2Nx-HP) with moderately increased in dietary phosphate and compared with Sham rats on the same diet. Additional in vitro studies were performed to evaluate the effect of FGF23 on osteocytes and osteoclasts formation. Our results show that serum FGF23 levels are increased in Nx1/2-HP without significant differences in other parameters of mineral metabolism such as PTH, phosphate or calcitriol. Bone histomorphometric analysis revealed that animals with high FGF23 had a decreased bone volume and a high bone turnover. RNA analysis of bones revealed a decrease in the expression of specific osteogenic genes such as Runx2, Osterix or DMP1 and an increase of SOST expression that was also detected in plasma.
In vitro, high rFGF23 was added during maturation of mesenchymal stem cells into mature osteoblasts or for 24 hours once mature. Alkaline phosphatase activity and osteoblast master genes expression were decreased in FGF23-treated cells. In mature osteocytes, high rFGF23 downregulated osteoblast gene expression: Osterix, Osteocalcin, DMP1 and RANKL. Furthermore, high FGF23 levels decreased the nuclear translocation of ß-catenin after 24 hours. With respect to osteoclasts formation, the presence of FGF23 enhanced TRAP staining, the number of nuclei and osteoclasts and cathepsin K expression. In conclusion, high FGF23 inhibited canonical Wnt signaling and osteoblast maturation.
4. Trabecular bone score in osteogenesis imperfecta. Is it useful?
Flórez H1, Muxi A2, González E3, Monegal A1, Guañabens N1, Peris P1
1 Metabolic Bone Diseases Unit. Department of Rheumatology. Hospital Clinic. University of Barcelona; 2 Department of Nuclear Medicine. Hospital Clinic. University of Barcelona; 3 Department of Immunology. Hospital Clinic. University of Barcelona
The trabecular bone score (TBS) is a novel gray-level textural analysis measurement that can be applied to DXA images to estimate trabecular microarchitecture and has been shown to be related to direct measures of bone microarchitecture and fracture risk. Osteogenesis imperfecta (OI) is a congenital bone disease characterised by a low bone mineral density (BMD) and poor bone quality and strength. The usefulness of TBS in OI has been scarcely evaluated.
Purpose: To analyse the clinical usefulness of TBS determination in patients with OI and its relation with anthropometric and clinical features (especially concerning skeletal fractures and BMD results).
Methods: Thirty-four patients (23F:11M) with a mean age of 40+15 years (19-70) attending a Metabolic Bone Disease Unit were included. The clinical reports of the patients were reviewed, with especial attention to the clinical features (weight, height and body mass index (BMI)), previous fractures, disease severity, associated mutations and treatments received. Lumbar spine (LS), total hip (TH), and femoral neck (FN) BMD were measured using DXA equipment (Lunar) in all patients. TBS was analysed in LS, and the results were classified in three categories1: TBS >1.310 (normal), TBS 1.230-1.310 (partially degraded microarchitecture), TBS <1.230 (degraded microarchitecture). TBS values were compared with a healthy control group of similar age and gender.
Results: 6/31 patients (19%) had a degraded microarchitecture, 8 (26%) a partially degraded microarchitecture and 17 (55%) normal TBS. All patients with TBS <1.230 werw over 40 years old. 97% had a previous history of fractures, most with multiple fractures. Regarding BMD, 61% of the patients had osteoporosis, 36% osteopenia and one had normal values. Most patients had mutations in the COL1A1 or COL1A2 genes (66% and 34%, respectively). No significant differences were observed in BMD or TBS values according to the COL1 gen mutation (COL1A1 vs. COL1A2). A correlation was observed between TBS and age (r=0.6, p<0.01), LS BMD (r=0.4, p=0.03), TH BMD (r=0.4, p=0.04) and with BMI (r=0.5, p=0.01). No significant differences were observed on comparing TBS in patients and controls (1.297 vs. 1.399, p=N.S.).
Conclusions: TBS measurement does not seem to be useful for evaluating bone strength in patiens with OI. Despite most patients presenting a history of multiple fractures, only 19% showed degraded microarchitecture with TBS.
1.- McCloskey EV. J Bone Miner Res. 2016.
5. Can DXA-derived 3D measurements at the lumbar spine predict thoracic spine fractures?
López Picazo Mu, Humbert L1, Di Gregorio S3, González Ballester MA2·4, Río L del3
1 Galgo Medical. Barcelona. Spain; 2 BCN MedTech. Universitat Pompeu Fabra. Barcelona. Spain; 3 CETIR Grup Mèdic. Barcelona. España; 4 ICREA. Barcelona. Spain
Objective: Evalúate the association of DXA-derived 3D measurements at the lumbar spine with thoracic spine fractures.
Method: We retrospectively analyzed a cohort of 32 postmenopausal Caucasian women collected at CETIR Grup Mèdic: 16 subjects with thoracic spine fractures (fracture group) and 16 agematched subjects without any type of fracture (control group). Inclusion criteria for the fracture group were no prior osteoporotic fractures at baseline, and thoracic spine fracture event between one year to ten years from baseline visit. Inclusion criteria for the control group were no prior fractures at baseline and during at least 7 years from baseline visit. Spine AP DXA scans were acquired at baseline using a Prodigy scanner (GE Healthcare). Areal BMD (aBMD) at L1-L4 segment was measured using enCORE software (GE Healthcare). DXA-derived 3D measurements at L1-L4 segment were assessed using the software 3D-SHAPER (Galgo Medical). The software computes the 3D shape and density distribution of the lumbar spine by registering a statistical model onto the AP DXA scan. Volumetric BMD (vBMD) is computed at trabecular, cortical and integral (cortical plus trabecular) compartments. Differences in aBMD and DXAderived 3D measurements between fracture and control groups were evaluated using unpaired ttest. Individual odds ratio (OR) and area under the receiver operating curve (AUC) were computed.
Results: No significant difference between groups was found in terms of age (ρ=0.74), weight (ρ=0.44), height (ρ=0.25), T-score (ρ=0.10), aBMD (ρ=0.11) and integral vBMD at the total vertebra (vertebral body plus posterior arch) (ρ=0.05). However, when computed at the vertebral body, integral, trabecular and cortical vBMD showed significant differences (Table I). Higher AUC were found for vBMD measurements at the vertebral body, compared to aBMD and integral vBMD at total vertebra. Each incremental decrease of one standard deviation of the aBMD was associated with 1.86 the odds of presenting a fracture at dorsal vertebrae. OR for trabecular vBMD at the vertebral body was 5.42.
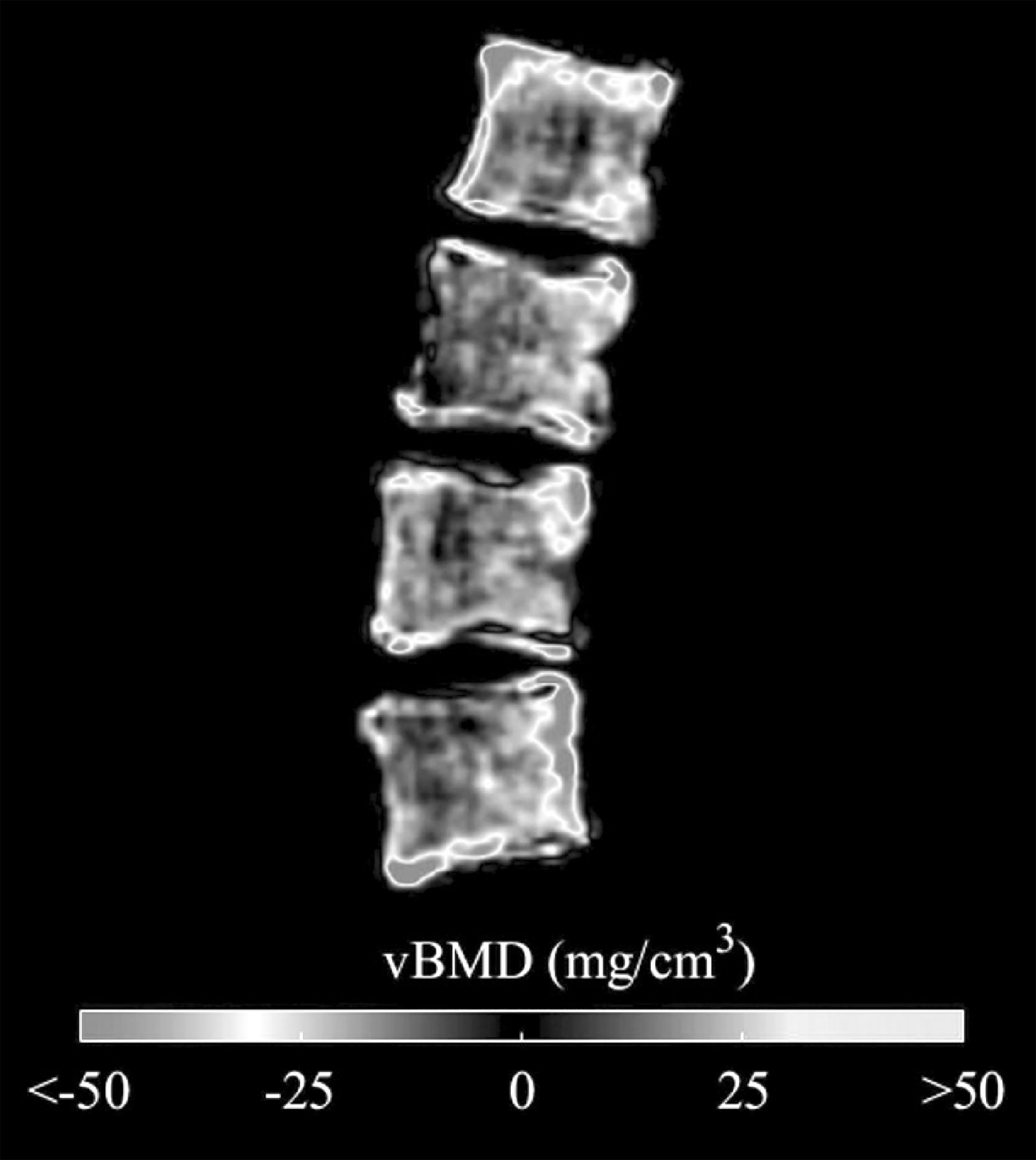
Figure Differences between average vBMD of each group showed at the mid sagittal plane of the vertebral body
Conclusion: This study shows that DXA-derived 3D measurements at the vertebral body could potentially be used to predict thoracic spine fracture using standard L1-L4 AP DXA scans.