Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista de Osteoporosis y Metabolismo Mineral
versão On-line ISSN 2173-2345versão impressa ISSN 1889-836X
Rev Osteoporos Metab Miner vol.11 no.1 Madrid Jan./Mar. 2019
https://dx.doi.org/10.4321/s1889-836x2019000100002
Originals
Calcidiol levels and muscle function maintenance, functional capacity and bone mineral bone density in non-selected Spanish population
1Unidad de Gestión Clínica de Metabolismo Óseo - Instituto de Investigación Sanitaria del Principado de Asturias (ISPA) - Red de Investigación Renal (REDinREN) del Instituto de Salud Carlos III - Universidad de Oviedo - Oviedo (España)
2Servicio de Medicina Interna - Hospital Universitario Central de Asturias - Oviedo (España)
3Laboratorio de Medicina - Hospital Universitario Central de Asturias - Oviedo (España)
4Unidad de Cuidados Intensivos - Hospital Universitario Central de Asturias - Oviedo (España)
Introduction:
Vitamin D offers beneficial effects that reportedly help maintain musculoskeletal function.
Aim:
To analyze the effect of calcidiol levels on muscle function in both hands, on activities of daily life and on changes in bone mineral density (BMD) in an unselected population.
Material and methods:
The EVOS study cohort was used, which carried out, among others, measures of muscular strength of grip in both hands, questions related to difficulty in performing daily activities, densitometric study in the lumbar and hip spine, and biochemistry to determine the levels of calcidiol.
Results:
Calcidiol values ≥20 ng/mL were associated with greater grip strength in both hands. After adjusting for age, sex, BMI and seasonality, calcidiol levels <20 ng/mL were independently associated with lower grip strength only in the left hand (OR=2.35; 95% CI: 1.03-5.38). Likewise, the inability or difficulty to "pick up a book or object from a high shelf" and "get up from the bed" were significantly associated with calcidiol levels <20 ng/mL. Levels of calcidiol <20 ng/mL were associated with greater BMD losses in the femoral neck and total hip. These associations were maintained in the multivariate analysis.
Conclusions:
Maintaining levels of calcidiol ≥20 ng/mL was associated with greater muscular strength of grip in the hands, maintenance of daily activities and lower BMD losses in the hip. This study corroborates the utility of maintaining adequate levels of vitamin D to maintain musculoskeletal function.
Key words: calcidiol; muscle strength; functional capacity; bone mineral density
INTRODUCTION
The aging process is associated with a loss of muscle mass and strength, as well as a decrease in bone mineral density (BMD), which can lead to reduced mobility, greater risk of falls and the appearance of fractures1,2. In recent years, special emphasis has been placed on maintaining an adequate vitamin D status to optimize muscle strength and BMD in order to reduce falls and fractures3-5. Although a recent meta-analysis questions the usefulness of vitamin D supplements to reduce the risk of falls, BMD decrease and fractures6, there are sufficient arguments that demonstrate the importance of vitamin D on muscle and bone health. Vitamin D stimulates the absorption of calcium from the intestine and maintains the serum calcium levels that are required for normal bone mineralization and for the maintenance of muscle function7. Several in vivo studies suggest vitamin D’s role in regulating muscle mass and its function. Observational studies show that vitamin D deficiency in the elderly is associated with reduced muscle mass and strength8-10, lower physical performance8,11, and increased risk of falls12. In addition, a meta-analysis of 17 clinical trials showed that supplementation with vitamin D in subjects with basal calcidiol levels below 10 ng/mL had a positive effect on hip muscle strength13. These studies suggest that vitamin D can affect muscle mass and function. However, it is not clear whether vitamin D plays a direct or indirect role. In recent years, the local conversion of calcidiol to calcitriol, the most active vitamin D metabolite, which is synthesized mainly in the kidney through its precursor calcidiol, has been increasingly important7. This local synthesis has been reported in several other cell types, such as in osteoblasts14-17, prostate cells18 and monocytes19, which reinforces the importance of reaching adequate levels of calcidiol in the body.
Therefore, the aim of our study was to analyze in an unselected population the effect of calcidiol (25-OHD) levels on muscle strength in both hands, activities of daily life related to the functional capacity of the individual and the changes in the BMD.
MATERIAL AND METHODS
The initial study protocol was designed to ascertain the prevalence of vertebral fracture. To do this, 624 men and women over 50 years of age were randomly selected from the municipal registry of Oviedo, Spain. The protocol con- sisted of all subjects completing a questionnaire on risk factors related to osteoporosis. This questionnaire was designed for the EVOS study, translated into several languages, and had an adequate reproducibility index20, 21. Similarly, the entire cohort underwent two lateral radiographs (this radiographic study was not completed in only two cases), the collection of anthropometric measurements such as height and weight to determine the body mass index (BMI), and a densitometric study. All subjects had sufficient ambulatory capacity to climb two floors without a lift and 99% lived in their own home.
After the prevalence study, this cohort was followed prospectively for 4 years by means of 2 postal questionnaires to find out the incidence of non-vertebral osteoporotic fracture. In the fourth year of the follow-up period (between the second and the third postal questionnaire), participants who had answered at least one of the two previous questionnaires were invited to repeat the same tests performed in the prevalence study, to which measures of muscular strength of grip in both hands were added to him with a dynamometer that owns a scale that goes from the minimum 0 to the maximum of 300 mm of Hg, a survey with 12 items on the difficulty or not to carry out daily life activities, as well as a biochemical study of general markers and bone and mineral metabolism. In this second cross-sectional study, 404 subjects participated (212 women and 192 men), of which 322 agreed to take part in the biochemical study. A total of 32 subjects (9.9%) were excluded from the analysis as they had undergone osteoporotic treatment, including treatment with vitamin D. From a total of 290 subjects, we had all the data in both cross sections.
Densitometric evaluation
The BMD was measured with a Hologic® QDR-1000 DXA densitometer (Hologic Inc., Waltham, Massachusetts, USA). In all cases, the anterior-posterior lumbar spine (L2-L4) and the density of the right femur were analyzed. For the evaluation of lumbar BMD, 4 subjects with marked degenerative osteoarthritis were excluded. The coefficients of variation (CV) were 1.2% and 1.9%, respectively22. The long-term daily quality control was followed by a phantom of the lumbar spine, with CV=0.0±0.1%20. In the fourth year of the follow-up period, BMD was also determined in the same areas as those measured in the first cross-sectional study, using the rate of change in BMD between both cross-sectional studies as a method to evaluate BMD development over time.
Biochemical analysis
Biochemical analysis In the fourth year of follow-up and over 1 year, a sample of blood and urine was taken in fasting from each subject: 33% of the blood samples were taken in the spring, 12% in the summer, 32% in the autumn and 23% in winter. Once the serum was separated, it was stored frozen together with the urine at -80ºC until the analyzes were carried out. Serum levels of calcium, creatinine, total alkaline phosphatase and resistant tartrate acid phospha-tase were determined using an autoanalyzer (Hitachi Mod. 717, Ratigen, Germany). The serum levels of calcidiol (25OHD) were determined by previous extraction with acetonitrile (IDS, Ltd., Bolton, United Kingdom), whose intra- and interassay coefficients of variation (CV) were, respectively, 5.2% and 8.2% respectively.
Levels of 1,25-dihydroxyvitamin D were measured by radio-immunoassay (IDS, Ltd.); the intra- and interassay CVs were 6.5% and 9%, respectively. Intact levels of PTH were measured using radio-immunoassay methods (Nichols Institute, San Juan de Capistrano, California, USA); the intra- and interassay CV values were 2.6% and 5.8%, respectively.
All the studies carried out followed the principles set out in the Helsinki Declaration and were formally approved by the Clinical Trials Committee of the Principality of Asturias.
Statistic analysis
The analysis of the data was carried out using version 17.0 of SPSS for Windows. The quantitative variables were analyzed by Student's t test. The qualitative variables analyzed by chi square.
To analyze at multivariate level the effect of calcidiol levels on muscle strength, the muscular strength of grip in both hands was categorized as 0 for values equal to 300 mm Hg (maximum pressure of the dynamometer) and 1 for values <300 mm of Hg. The logistic regression analysis was adjusted for age, sex, BMI and seasonality (season of the year in which blood extraction was carried out).
To study the association between the performance of daily life activities with serum levels of calcidiol, a logistic regression analysis was carried out after adjusting for age, sex, BMI and seasonality.
When statistically significant associations were found between the levels of calcidiol and the rate of change in BMD in the univariate analysis, a linear regression was performed adjusted for age, sex, BMI and seasonality.
RESULTS
Table 1 shows sociodemographic, anthropometric, clinical variables and biochemical markers of the cohort analyzed as a function of serum levels of calcidiol. In those with calcidiol levels of ≥20 ng/mL, there was a predominance of men, younger age, higher BMD values in all the skeletal segments analyzed, lower frequency of previous fractures, higher levels of calcitriol and lower levels of PTH and total alkaline phosphatase.
Table 1. Demographic, anthropometric characteristics, clinical variables and biochemical markers as a function of serum levels of calcidiol

Calcidiol values =20 ng/mL (28.6% of the cohort) were associated with greater grip strength in both hands compared to levels <20 ng/mL (Figure 1). After adjusting for age, sex, BMI and seasonality, calcidiol levels <20 ng/mL alone were associated independently with decreases in grip strength (OR=2.35; 95% CI: 1.03-5.38). On the other hand, that association was lost in the right hand (OR=1.91; 95% CI: 0.92-3.98).

Figure 1. Grip strength measurements (mm Hg) in hand A) left; B) right according to the serum levels of calcidiol. *p <0.05 for calcidiol <10 ng/mL and calcidiol between 10-20 ng/mL
The daily life activities as a function of calcidiol levels are reflected in table 2. Of the 12 activities analyzed, the inability or difficulty to "lean to catch a soil object" was significantly associated with lower levels of calcidiol (p=0.009, table 2).
Table 2. Levels of calcidiol (ng/mL) depending on the difficulty or not to perform certain activities of daily life

Likewise, the difficulty or inability to: "get out of bed" was associated with lower levels of calcidiol; "Pick up a book or object from a high shelf"; "Leaning from a chair to take object from the floor"; "Remove the stockings or socks" and "run 100 meters without stopping" (Table 2). Only "bending down to get an object from the floor" and "getting up from the bed" were significantly associated with calcidiol levels after multivariate adjustment for age, sex, BMI and seasonality. Thus, increments of 10 ng/mL of calcidiol were associated with a decrease of 30% and 58%, respectively, in the difficulty or inability to "bend over to pick up an object from the floor" or to "get up from bed".
The stratification of calcidiol levels showed that, in the multivariate adjustment, the presence of calcidiol deficiency (<10 ng/mL) not only significantly increased the inability or difficulty to "get out of bed: (OR=2.14; 95% CI: 1.21-3.77)" but also to "take a book or object from a high bookshelf: (OR=2.02; 95% CI: 1.09-3.73)", "lean from a chair to take object from the floor: (OR=1.78; 95% CI: 1.03-3.07)" and "remain seated in a hard chair for 1 hour: (OR=1.78; 95% CI: 1.03-3.07)".
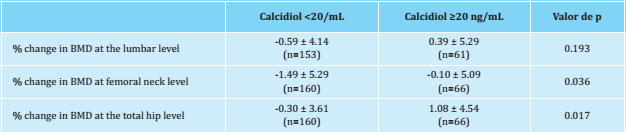
The percentage of change in BMD at the level of the lumbar spine, femoral neck and total hip as a function of the serum levels of calcidiol is shown in table 3. The presence of calcidiol levels <20 ng/mL was associated with greater losses of BMD, both at the femoral neck and total hip level, with no significant differences at the lumbar level. After a multivariate analysis, changes in BMD at femoral neck and total hip level were independently associated with calcidiol levels <20 ng/mL (typified beta coefficient=0.130, p=0.041 and typified beta coefficient=0.142, p=0.033, respectively).
DISCUSSION
In this study, low levels of calcidiol (<20 ng/mL) have been found to contribute to a lower muscular strength of grip in the hands, to more difficulties to perform certain activities of daily life, as well as to greater losses of BMD in the hip.
There are both basic and clinical evidences that support the participation of vitamin D in skeletal muscle function23. In recent work in people with spinal cord injuries requiring rehabilitation, low levels of calcidiol were independent predictors of decreased physical function24. The lower muscle strength of grip in relation to the low levels of calcidiol found in our study has also been reported by other authors25. Thus, in a longitudinal study of Dutch adults, aged between 55 and 85 years, serum levels of calcidiol below 10 ng/mL were associated with a 40% loss in grip strength compared to baseline26.
In our study, some activities of daily life were found to be compromised by the low levels of calcidiol, this effect being more marked in the presence of calcidiol deficiency (<10 ng/mL). The activities that were most affected were those that had more to do with the functional capacity of the organism than those dependent on greater muscular strength such as "transporting an object of 10 kg for 10 meters" or "lifting a box with 6 full bottles and place them on a table." Other recent studies have also associated low levels of vitamin D with the greatest difficulty in performing activities of daily living. Thus, in a recent study by Arbex Borim et al., the reduction of muscle strength or dynapenia combined with low levels of calcidiol were found to be a risk factor that conditioned the development of daily life activities in a sample of 4,630 people over 50 free of disability at the outset of the study, followed for 2 years27. Similarly, Wicherts et al., analyzing a study of men and women between 65 and 88 years old, found that those with calcidiol levels below 20 ng/mL had a worse state and physical performance at both baseline and 3 years of follow-up compared to those with levels higher than 30 ng/mL11. Another Dutch prospective study showed that vitamin D levels were associated with functional limitations in the age stratum between 55 to 65 years and in those over 65 years28. However, others authors have not found any association between calcidiol levels below 10 ng/mL and lower hip flexion, knee extension force, grip strength, gait speed or disability in activities related to mobility of the upper extremities. This last study was carried out in 628 women over 65 years of age followed for 3 years and who presented a moderate to severe disability at the beginning of the study. The existence of very few participants with low calcidiol values may have limited the possibility of obtaining differences29.
The association between levels of calcidiol and BMD revealed in a recent meta-analysis is more contradictory6. Epidemiological evidence indicates that the highest levels of calcidiol are associated with higher BMD in both the young and aging population, maintaining a linear relationship to levels of 30 ng/mL, an association that does not seem so clear and solid in black populations. or Hispanics of North America30. Our data indicate a direct association between calcidiol levels <20 ng/mL and BMD at femoral neck and total hip level. A 2014 meta-analysis concluded that there was very little evidence that vitamin D influenced BMD, since there was no consistent relationship between vitamin D supplementation and BMD in most of the anatomical sites analyzed (lumbar spine, total hip, trochanter, whole body or forearm), although a positive association was observed in the femoral neck, as was observed in our study31. Similarly, a recent article shows that patients with hip fractures have lower levels of calcidiol, lower bone mass, decreased bone quality and an increased risk of fracture32. It is important to highlight that in our study all subjects who were receiving treatment for osteoporosis, including supplements with vitamin D, were eliminated, which does not allow us to assess the possible effect of vitamin D supplementation on bone mass.
Our study has limitations, but also strengths. Regar ding the former, the fact of having a single biochemical determination (after 4 years of follow-up) without knowing the values at the beginning of the study limits the associations found. On the other hand, the questionnaire on difficulties to carry out activities of daily life was not self-administered but administered by an interviewer, which could have biased the responses of the participants, especially in those questions that referred to personal hygiene difficulties. As strengths, the analyzed cohort participated in the EVOS-EPOS study, being one of the few groups that completed all the study guidelines. The participation percentages of more than 80% in the four postal follow-ups carried out during 8 years guarantee the representativeness of the sample analyzed. In addition to the articles published with data from the full cohort of the EVOS-EPOS study, the cohort of the city of Oviedo, which has been used for this study, has contributed individually to the publication of several original articles in high-impact journals33- 39.
To sum up, calcidiol levels above 20 ng/mL are asso ciated with greater muscle grip strength in the hands, better performance in activities of daily life such as "bending over to pick up an object from the ground" and "Get up from the bed" and with a greater BMD in total hip and femoral neck, suggesting that maintaining calcidiol levels above 20 ng/mL would favor an adequate musculoskeletal function.
REFERENCES
1 Cederholm T, Cruz-Jentoft AJ, Maggi S. Sarcopenia and fragility fractures. Eur J Phys Rehabil Med. 2013;49:111-7. [ Links ]
2 Rizzoli R, Stevenson JC, Bauer JM, van Loon LJ, Walrand S, Kanis JA, et al. The role of dietary protein and vitamin D in maintaining musculoskeletal health in postmenopausal women: A consensus statement from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Maturitas. 2014;79:122-32. [ Links ]
3 Morgan KT. Nutritional determinants of bone health. J Nutr Elder. 2008;27:3-27. [ Links ]
4 Lips P, van Schoor NM. The effect of vitamin D on bone and osteoporosis. Best Pract Res Clin Endocrinol Metab. 2011;25:585-91. [ Links ]
5 Bischoff-Ferrari HA. Relevance of vitamin D in muscle health. Rev Endocr Metab Disord. 2012;13:71-7. [ Links ]
6 Bolland MJ, Grey A, Avenell A. Effects of vitamin D supplementation on musculoskeletal health: a systematic review, meta-analysis, and trial sequential analysis. Lancet Diabetes Endocrinol. 2018;6:847-58. [ Links ]
7 Lips P. Vitamin D physiology. Prog Biophys Mol Biol. 2006;92:4-8. [ Links ]
8 Tieland M, Brouwer-Brolsma EM, Nienaber-Rousseau C, van Loon LJ, de Groot LC. Low vitamin D status is associated with reduced muscle mass and impaired physical performance in frail elderly people. Eur J Clin Nutr. 2013;67:1050-5. [ Links ]
9 Bischoff HA, Stahelin HB, Urscheler N, Ehrsam R, Vonthein R, Perrig-Chiello P, et al. Muscle strength in the elderly: Its relation to vitamin D metabolites. Arch Phys Med Rehabil. 1999;80:54-8. [ Links ]
10 Zamboni M, Zoico E, Tosoni P, Zivelonghi A, Bortolani A, Maggi S, et al. Relation between vitamin D, physical performance, and disability in elderly persons. J Gerontol A Biol Sci Med Sci. 2002;57:M7-11. [ Links ]
11 Wicherts IS, van Schoor NM, Boeke AJ, Visser M, Deeg DJ, Smit J, et al. Vitamin D status predicts physical performance and its decline in older persons. J Clin Endocrinol Metab. 2007;92:2058-65. [ Links ]
12 Snijder MB, van Schoor NM, Pluijm SM, van Dam RM, Visser M, Lips P. VitaminD status in relation to one-year risk of recurrent falling in older men and women. J Clin Endocrinol Metab. 2006;91:2980-5. [ Links ]
13 Stockton KA, Mengersen K, Paratz JD, Kandiah D, Bennell KL. Effect of vitamin D supplementation on muscle strength: A systematic review and meta-analysis. Osteoporos Int. 2011;22:859-71. [ Links ]
14 Howard GA, Turner RT, Sherrard DJ, Baylink DJ. Human bone cells in culture metabolize 25-hydroxyvitamin D3 to 1, 25-dihydroxyvitamin D3 and 24, 25-dihydroxyvitamin D3. J Biol Chem. 1981;256:7738-40. [ Links ]
15 van Driel M, Koedam M, Buurman CJ, Hewison M, Chiba H, Uitterlinden AG, et al. Evidence for auto/paracrine actions of vitamin D in bone: 1ahydroxylase expression and activity in human bone cells. FASEB J. 2006;20:2417-9. [ Links ]
16 Atkins GJ, Anderson PH, Findlay DM, Welldon KJ, Vincent C, Zannettino AC, et al. Metabolism of vitamin D3 in human osteoblasts: Evidence for autocrine and paracrine activities of 1a, 25-dihydroxyvitamin D3. Bone. 2007;40:1517-28. [ Links ]
17 van der Meijden K, Lips P, van Driel M, Heijboer AC, Schulten EA, den Heijer M, et al. Primary human osteoblasts in response to 25-Hydroxyvitamin D3, 1, 25-Dihydroxyvitamin D3 and 24R, 25-Dihydroxyvitamin D3. PLoS ONE. 2014;9:e110283. [ Links ]
18 Schwartz GG, Whitlatch LW, Chen TC, Lokeshwar BL, Holick MF. Human prostate cells synthesize 1, 25-dihydroxyvitaminD3 from 25-hydroxyvitaminD3. Cancer Epidemiol Biomarkers Prev. 1998;7:391-5. [ Links ]
19 Bacchetta J, Sea JL, Chun RF, Lisse TS, Wesseling-Perry K, Gales B, et al. Fibroblast growth factor 23 inhibits extrarenal synthesis of 1,25- dihydroxyvitamin D in human monocytes. J Bone Miner Res. 2013;28:46-55. [ Links ]
20 O´Neill TW, Cooper C, Algra D, Pols HAP, Agnusdei D, Dequeker J, et al, on behalf of the European Vertebral Osteoporosis Study Group. Design and development of a questionnaire for use in a multicentre study of vertebral osteoporosis in Europe: The European vertebral osteoporosis study (EVOS). Rheumatol Eur. 1995;24:75-81. [ Links ]
21 O'Neill TW, Cooper C, Cannata JB, Diaz Lopez JB, Hoszowski K, Johnell O, et al, on behalf of the European Vertebral Osteoporosis Study (EVOS) Group. Reproducibility of a questionnaire on risk factors for osteoporosis in a multicentre prevalence survey: the European Vertebral Osteoporosis Study. Int J Epidemiol. 1994;23:559-65. [ Links ]
22 Gómez Alonso C. Valores de la densidad mineral ósea (BMD) en columna lumbar y cadera de la población sana española. En: Díaz Curiel M, Díez Pérez A, Gómez Alonso C, FHOEMO-SEIOMM-RPR (eds). Nuevas fronteras en el estudio de la densidad ósea en la población española. Madrid: Rhone Poulenc Rorer; 1996. p. 73-94. [ Links ]
23 Girgis CM, Clifton-Bligh RJ, Hamrick MW, Holick MF, Gunton JE. The roles of vitamin D in skeletal muscle: form, function, and metabolism. Endocr Rev. 2013;34:33-83. [ Links ]
24 Barbonetti A, D´Andrea S, Martorella A, Felzani G, Francavilla S, Francavilla F. Low vitamin D levels are independent predictors of 1-year worsening in physical function in people with chronic spinal cord injury: a longitudinal study. Spinal Cord. 2018;56:494-501. [ Links ]
25 Nicoletti Gumieiro D, Murino Rafacho BP, Buzati Pereira BL, Alvisi Cavallari K, Erico Tanni S, Schmidt Azevedo P, et al. Vitamin D serum levels are associated with handgrip strength but not with muscle mass or length of hospital stay after hip fracture. Nutrition. 2015;31:931-4. [ Links ]
26 Visser M, Deeg DJH, Lips P. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): The Longitudinal Aging Study Amsterdam. J Clin Endocrinol Metab. 2003;88:5766 e 5772. [ Links ]
27 Arbex Borim FS, da Silva Alexandre T, Liberalesso Neri A, de Oliveira Máximo R, Fernandes Silva M, de Oliveira C. Combined effect of dynapenia (muscle weakness) and low vitamin D status on incident disability. J Am Med Dir Assoc. 2019;20:47-52. [ Links ]
28 Sohl E, van Schoor NM, de Jongh RT, Visser M, Deeg DJH, Lips P. Vitamin D status is associated with functional limitations and functional decline in older individuals. J Clin Endocrinol Metab. 2013;98:E1483-90. [ Links ]
29 Verreault R, Semba RD, Volpato S, Ferrucci L, Fried LP, Guralnik JM. Low serum vitamin D does not predict new disability or loss of muscle strength in older women. J Am Geriatr Soc. 2002;50: 912-7. [ Links ]
30 Bischoff-Ferrari HA, Dietrich T, Orav EJ, Dawson-Hughes B. Positive association between 25-hydroxy vitamin D levels and bone mineral density: a population-based study of younger and older adults. Am J Med. 2004;116:634-9. [ Links ]
31 Reid IR, Bolland MJ, Grey A. Effects of 2014 vitamin D supplements on bone mineral density: a systematic review and meta-analysis. Lancet. 2014;383:146-55. [ Links ]
32 Montoya MJ, Vázquez MA, Miranda C, Miranda MJ, Pérez-Cano R, Giner M. Influencia de la vitamina D sobre la microestructura y propiedades biomecánicas de pacientes con fractura de cadera. Rev Osteoporos Metab Miner. 2017;9:121-9. [ Links ]
33 Gómez C, Naves ML, Barrios Y, Díaz JB, Fernández JL, Salido E, et al. Vitamin D receptor gene polymorphisms, bone mass, bone loss and prevalence of vertebral fracture: differences in postmenopausal women and men. Osteoporos Int. 1999;10:175-82. [ Links ]
34 Naves M, Díaz-López JB, Gómez C, Rodríguez-Rebollar A, Rodríguez-García M, Cannata-Andía JB. The effect of vertebral fracture as a risk factor for osteoporotic fracture and mortality in a Spanish population. Osteoporos Int. 2003;14:520-4. [ Links ]
35 Naves M, Díaz-López JB, Gómez C, Rodríguez-Rebollar A, Serrano-Arias M, Cannata-Andía JB. Prevalence of osteoporosis in men and determinants of changes in bone mass in a non-selected Spanish population. Osteoporos Int. 2005;16:603-9. [ Links ]
36 Naves M, Díaz-López JB, Gómez C, Rodríguez-Rebollar A, Cannata-Andía JB. Determinants of incidence of osteoporotic fractures in the female Spanish population older than 50. Osteoporos Int. 2005;16:2013-7. [ Links ]
37 Naves M, Rodríguez-García M, Díaz-López JB, Gómez-Alonso C, Cannata-Andía JB. Progression of vascular calcifications is associated with greater bone loss and increased bone fractures. Osteoporos Int. 2008;19:1161-6. [ Links ]
38 Naves-Díaz M, Cabezas-Rodríguez I, Barrio-Vázquez S, Fernández E, Díaz-López JB, Cannata-Andía JB. Low calcidiol levels and risk of progression of aortic calcification. Osteoporos Int. 2012;23:1177-82. [ Links ]
39 Tuñón-Le Poultel D, Cannata-Andía JB, Román-García P, Díaz-López JB, Coto E, Gómez C, et al. Association of matrix Gla protein gene functional polymorphisms with loss of bone mineral density and progression of aortic calcification. Osteoporos Int. 2014;25:1237-46. [ Links ]
This study was partially fundedby the European Study on Vertebral Osteoporosis(EVOS), European Union (1991-1993); EuropeanProspective Study on Osteoporosis (EPOS), European Union (BIOMED 93-95), BMHI-CT 092-0182(1993-1997); Sanitary Research Fund (FIS 94/1901-E); RETIC REDINREN of the ISCIII - European Regional Development Fund (RD06/0016/1013, RD12/0021/1023 and RD16/0009/0017); National R+D+IPlan 2008-2011, State R+D+ I Plan 2013-2016, Carlos III Health Institute (ISCIII); Plan of Science, Technology and Innovation 2013-2017 of the Principalityof Asturias (GRUPIN14-028); Foundation for thePromotion of Applied Scientific Research and Technology in Asturias (FICYT) and Asturian Society forthe Development of Metabolic Research.
Received: November 16, 2018; Accepted: January 29, 2019











 texto em
texto em