Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista de Osteoporosis y Metabolismo Mineral
versão On-line ISSN 2173-2345versão impressa ISSN 1889-836X
Rev Osteoporos Metab Miner vol.11 no.1 Madrid Jan./Mar. 2019
https://dx.doi.org/10.4321/s1889-836x2019000100003
Originals
Effects of mechanical stimulation on communication between bone cells
1Instituto de Medicina Aplicada de la Universidad San Pablo-CEU - Madrid (España)
2Departamento de Ciencias Médicas Básicas - Facultad de Medicina - Universidad San Pablo CEU - Madrid (España)
Mechanical force is important for modeling, remodeling and bone regeneration. It stimulates the osteocytes, causing an alteration in the production and secretion of signaling molecules that regulate osteoblast and osteoclast activity. The present study aims to evaluate the effect of the conditioned medium of mechanically stimulated mouse osteocytic cells on the proliferative and migratory capacity of mesenchymal cells and bone cells. For this, the proliferation and migration of mouse pre-osteoblastic cells, human pre-adult mesenchymal cells and mouse macrophages in the presence of the conditioned medium of osteocytic cells were analyzed, after 6 and 24 hours after being subjected to a mechanical stress of 10 dynes/cm2 by fluid flow (FF) for 10 minutes. The migration of pre-osteoblastic cells has been found to increase significantly in the presence of conditioned media of osteocytic cells compared to the static control group (SC) (SC=12.63±5.44, FF6h=23.03±11.57, FF24h=29.72±15.76, p<0.0001). In the same way, the pre-adipose cells also significantly increased their migration in the presence of this conditioned media (SC=11.48±4.75, FF6h=18.43±9.94, FF24h=18.80±10.03; p≤0.0007). However, macrophage migration decreased in the presence of the conditioned medium collected at 24 hours with respect to the static control group (SC=69±22.71, FF24h=26.57±5.47, p<0.0001). These effects were associated with decreased protein expression of certain chemokines, such as the monocyte chemotactic protein type I (SC=0.25±0.06, FF24h=0.09±0.005, p=0.0262), the protein of group I of high mobility (SC=0.25±0.04, FF24h=0.15±0.05, p=0.0159) and the regulatory protein of the activation of T lymphocytes and monocytes (SC=3.29±0.88, FF6h=1.33±1.09, FF24h=0.97±0.66, p≤0.0314), by the osteocytes in the presence of mechanical stimulation with respect to the static control group. In conclusion, this in vitro study demonstrates that osteocyte mechanotransduction enhances recruitment of osteoblasts and pre-adipose mesenchymal cells while reducing the migration of macrophages.
Key words: osteocytes; osteoblasts; macrophages; mesenchymal cells; mechanical stimulation; chemokines
INTRODUCTION
Mechanical force is one of the most important stimuli that the bone receives to regulate bone mass, shape and microarchitecture. The endoskeleton reacts to an increase in load by forming more bone or decreasing its mass in the absence of mechanical stress1. This is because the stimulation triggers the mechanotransduction process in which osteocytes, considered bone´s key mechanosensory cells, when stimulated, send chemical signals that affect the paracrine regulation of osteoblast and osteoclast behavior2,3. It also has been found to have an anti-apoptotic effect on osteocytes4.
With mechanical loading, the expression of sclerostin, which is an inhibitor of the protein signaling pathway Wnt/β-catenin constitutively secreted by osteocytes, decreases thus causing an increase in osteoblastogenesis5,6. On the other hand, apoptotic osteocytes induce the secretion of the receptor activator for nuclear factor κ B ligand (RANKL), indirectly stimulating osteoclastogenesis 7. In addition, some chemokines, a family of chemotactic cytokines, could be involved in bone remodeling when expressed by bone cells and provide key signals to recruit different cellular subpopulations8.
Recent studies indicate that the high mobility group box 1 protein (HMGB1), the regulated upon activation, normal T cell expressed, and secreted protein or chemokine (C-C motif) ligand 5 (RANTES or CCL5) and the monocyte chemoattractant protein 1 or chemokine (C-C motif) ligand 2 (MCP1 or CCL2) intervene to recruit mesenchymal stem cells to promote tissue repair9,10.
Based on this evidence, our objectives are focused on recreating an in vitro charge model to generate mechanotransduction in a controlled culture environment11 and to study the effect of conditioned medium secreted by osteocytes after being mechanically stimulated in the promotion of the proliferative and migratory capacity of mesenchymal cells and bone cells as well as the possible protein expression of certain chemotactic factors involved in proliferation and migration processes.
MATERIALS AND METHODS
Cell cultures. For our assays, different cell types were used:
Adipose stromal cells (ASC), obtained by primary culture of human lipoaspirates carried out in the HM Montepríncipe Hospital (HM Hospitals), as described in the work of Zuk et al. in 200112. All donors gave their informed consent, in accordance with the appropriate clinical protocol. The patients were operated in the Department of Plastic Surgery of HM Hospitals (Madrid, Spain), and the tissue sample collection was approved by the Institutional Review Board/Clinical Research Ethics Committee of HM Hospitals (Madrid, Spain). These cells were cultured with DMEM (Dulbecco modified Eagle´s minimal essential medium) + GlutaMAX (Gibco, Life Technologies, Alcobendas, Spain) with 10% fetal bovine serum (fetal bovine serum, FBS) and 1% penicillin-streptomycin (Invitrogen) at 37ºC with 5% CO2.
Continuous line of MLO-Y4 osteocytes from murine long bones extracted as described in Kato et al. in 1997, courtesy of L. Bonewald13, which was cultivated in 100 mm diameter plate (Jet Biofil, Guangzhou, China) previously collagenized with Collagen I (Sigma-Aldrich) with α MEM (Minimum Essential Medium Eagle - Alpha Modification) at 2.5% calf serum (Calf serum, CS) (Sigma-Aldrich), 2.5% FBS and 1% penicillin-streptomycin at 37ºC with 5% CO2.
Continuous line of bone mouse preosteoblast of the skull vault, MC3T3-E1 subclone 4 (ATCC CRL-2593).
Continuous line of mouse macrophages capable of differentiating to osteoclasts, RAW 264.7 (ATCC TIB-71), which were cultured with α MEM with 10% FBS, 1% penicillin-streptomycin and 2 mM L-glutamine at 37ºC with 5% CO2.
Mechanical stimulation tests by fluid passage (Fluid Flow, FF). This technique generates physiologically relevant mechanical stimulation in bone cells in vitro11. For this, 250,000 MLO-Y4 cells were seeded on Teflon-bound glass slides leaving a space of 15 cm2 previously collagenized and incubated for at least 48 hours at 37ºC with 5% CO2 until they reached the confluence. Subsequently, the cells were subjected to mechanical stimulation or not (static control or SC) with the Flexcell Streamer device of medium cut stress that produces a stress of 10 dynes/cm2 for 10 minutes (Flexcell International Corporation, Hillsborough, North Carolina, USA.). The cells were then incubated with α MEM Medium without phenol red (Gibco) with 0.5% CS, 0.5% FBS and 1% penicillin-streptomycin to obtain conditioned media (CM) from the different experimental groups: CM of stimulated cells (FF) collected at 6 hours after the stimulus, CM of SC cells collected at 24 hours after the stimulus and CM of FF cells collected at 24 hours after the stimulus.
Proliferation assay. To carry out the proliferation assay, both mouse pre-osteoblast cells and human pre-adipose cells were seeded at a concentration of 6,000 cells/well in 12-well culture plates (Jet Biofil), one plate per condition with each of the lines Cells, and incubated at 37ºC with 5% CO2. The following day, the medium was exchanged for 20% of conditioned medium and 80% of its culture medium, adjusting the FBS to 10%. After 24 hours of incubation at 37ºC with 5% CO2, the cells were raised with Trypsin-EDTA and a cell count was made with Trypan Blue 0.4% in PBS (GE Healthcare, Hyclone, Logan, Utah, USA) in the Neubauer chamber. The process was repeated at 48 and 72 hours, obtaining a proliferation assessment with each of the conditioned media for 3 days and in triplicate.
Migration trial in Transwell. Seed in 4 Transwell 6 well culture plates (Corning, Costar, Life Sciences, New York, USA) 75,000 cells/well with its culture medium on the membrane, and 20% conditioned medium was placed underneath and 80% of your culture medium at 1% FBS. After 24 hours of incubation at 37ºC with 5% CO2, both media and the upper cell layer of the membrane were removed with the aid of a cotton swab. The cells remaining at the bottom of the membrane were fixed with 4% paraformaldehyde in PBS (Alfa Aesar, Thermo Fisher) for 10 minutes and stained with 0.1% crystal violet in distilled water (MERCK, Kenilworth, New Jersey, USA) for 15 minutes. Finally, the membranes were mounted on slides and observed in the phase contrast microscope (Leica Microsystems DM5500 CTR6000) from which 20 images were obtained at 50 μm per well to analyze the number of cells that had migrated as a function of the conditioned medium used.
Western Blot. The cells were prepared to extract the total protein with RIPA buffer (Sigma-Aldrich) supplemented with protease inhibitors and phosphatases (Calbiochem). On the other hand, the conditioned medium was lyophilized and the pellet was resuspended in MiliQ water. To quantify the amount of protein in each of the samples, both lyophilized conditioned medium and cell lysate, the Varioskan Flash Multimode Reader (Thermo Scientific) with a Comassie template were used. Once quantified, they were separated in 15% acrylamide gels and transferred to nitrocellulose membranes. The membrane was then blocked with 5% bovine serum albumin (BSA) dissolved in TBS with Tween 20 (Sigma-Aldrich) for one hour at room temperature and incubated overnight at 4ºC with polyclonal antibodies. of rabbit: anti-HMGB1, anti-MCP1 and anti-RANTES (Abcam, Cambridge, UK). As a control, the anti-α tubulin mouse monoclonal antibody was used. It was then incubated for one hour at room temperature with the corresponding IgG coupled to peroxidase and the membrane was revealed in the transilluminator (Syngene DYV 6-E) with the ECL system (Electro-chemo-luminescence, GE-Amersham, Pittsburgh, USA). The intensities of the bands were quantified by densitometry.
Statistic analysis. In the statistical analysis of the results, the data are expressed as mean ± standard deviation of at least two experiments carried out in triplicate. It was performed using the GraphPad Prism V 7.0 software (GraphPad software, La Jolla, California, USA), using a non-parametric study using a two-tailed t-test or U-Mann-Whitney test for two-to-two comparisons, and the Kuskal-Wallis test for group comparisons. Outliers were detected and excluded using the GraphPad QuickCalcs©2018 program that uses the Grubb test, and values of p<0.05 were considered as significant results.
RESULTS
Effect of conditioned media of osteocytes mechanically stimulated in the proliferation of pre-osteoblasts and pre-adipose mesenchymal cells
A proliferation study of mouse pre-osteoblastic cells MC3T3-E1 and pre-adipose mesenchymal cells was performed with 20% conditioned media of mouse osteocytic cells MLO-Y4 in the presence (FF 6 hours and FF 24 hours) and absence (SC or control static) of mechanical stimulus by fluid passage.
As shown in figure 1A, there is no significant difference in the proliferation of the cell line MC3T3-E1 after 24 hours or after 72 hours in the presence of conditioned media of 6 and 24 hours. In the case of the pre-adipose mesenchymal cells, the results also showed no significant differences after 24 hours or after 72 hours in the presence of the same conditioned media (Figure 1B).

Figure 1. Cell proliferation of MC3T3-E1 (A) and ASC (B) (mesenchymal cells of adipose origin) in the presence and absence of conditioned media of 6 and 24 hours after mechanical stimulation. The values are the mean ± standard deviation of 3 independent experiments in triplicate. Results presented as number of cells vs. control
Effect of conditioned media of osteocytes mechanically stimulated in the migration of pre-osteoblasts, preadipose mesenchymal cells and macrophages
The study of migration of pre-osteoblastic cells MC3T3-E1, mesenchymal pre-adipose and macrophage cells RAW 264.7 was performed with culture medium specific to each cell line (control) and conditioned media of osteocytic MLO-Y4 cells in the presence (FF 6 hours and FF 24 hours) and absence (SC) of mechanical stimulation.
The pre-osteoblastic cells doubled and tripled their migration in the presence of the conditioned media of the osteocytes collected after 6 and 24 hours of being subjected to stimuli by fluid passage, respectively (Figure 2).

Figure 2. Migration of MC3T3-E1 cells. Representative images corresponding to the migration of MC3T3-E1 in each of the study conditions (A-C). Number of cells per field of MC3T3 migration in the absence and presence of conditioned study media (D). The values are the mean ± standard deviation of 2 independent experiments in triplicate. **p<0.001 vs. static control
In the same way, the pre-adipose mesenchymal cells also duplicated their migration in the presence of that media (Figure 3).

Figure 3. Migration of ASC cells (mesenchymal cells of adipose origin). Representative images corresponding to the migration of ASC in each of the study conditions (A-C). Number of cells per field of ASC migration in the absence and presence of conditioned study media (D). The values are the mean ± standard deviation of 2 independent experiments in triplicate. **p<0.001 vs. static control
In the case of the RAW 264.7 mouse macrophage line, our results indicate a three-fold decrease in their migration in the presence of conditioned media collected after 24 hours of performing the Fluid Flow (Figure 4).

Figure 4. Migration of RAW 264.7 cells. Representative images corresponding to the migration of RAW 264.7 in each of the study conditions (A-C). Number of cells per field of RAW migration 264.7 in the absence and presence of conditioned study media (D). The values are the mean ± standard deviation of 2 independent experiments in triplicate. **p<0.001 vs. static control
Analysis of chemokine expression and secretion after mechanically stimulating osteocytes
In order to corroborate the results obtained previously, the analysis of chemoattractant protein expression was carried out using the Western Blot technique. For this, the lysates of osteocytic cells MLO-Y4 were obtained in the presence and absence of mechanical stimulation by the passage of fluid and, on the other hand, the lyophilisation of their respective conditioned media, as described in the section on materials and methods.
We studied three possible proteins involved in the migration of mesenchymal cells after a mechanical stimulus, two of them belonging to the C-C chemokine family: MCP1 and RANTES, and the high mobility protein group 1 (HMGB1). Tubulin was used to normalize the cell lysate samples.
As seen in figure 5, in the cell lysates of mechanically stimulated MLO-Y4 there was a two-fold decrease in the expression of the chemotactic protein MCP1 (Figures 5A-5B). In the samples of lyophilized conditioned media it was observed that the secretion of MCP1 also decreased under the conditions of mechanical stimulation, in this case it decreased three times (Figures 5C-5D).
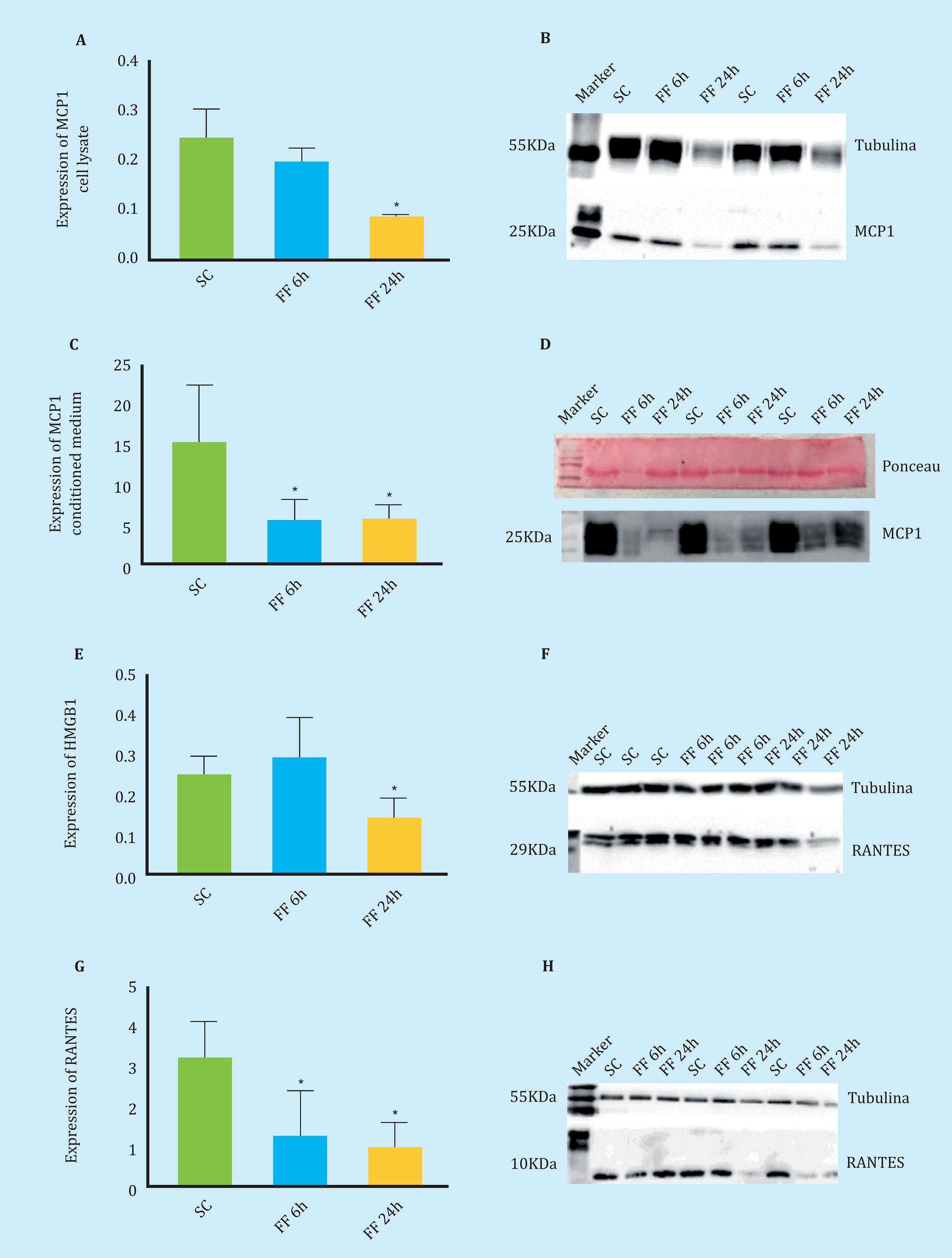
Figure 5. Secretion and expression of chemotactic proteins measured by Western Blot (A-C). Expression of the MCP1 protein in MLO-Y4 cell lysate in the absence of mechanical stimulation (SC) and after 6 and 24 hours of performing FF for 10 minutes (FF6h and FF24h) (C-D). Secretion of the MCP1 protein in conditioned MLO-Y4 media in the absence and presence of mechanical stimulus (E-F). Expression of HMGB1 in cell lysate of MLO-Y4 in the absence and presence of mechanical stimulus (G-H). Expression of RANTES in cell lysate of MLO-Y4 in the absence and presenceof mechanical stimulation. The relative densitometric values are the mean ± standard deviation of 2 independent experiments in triplicate. *p<0.05 vs. static control
In mechanically stimulated MLO-Y4 cell lysates, a two-fold decrease in the expression of the HMGB1 chemotactic protein was observed in the FF condition at 24 hours (Figures 5E-5F).
Similarly, in mechanically stimulated MLO-Y4 cell lysates, a threefold decrease in the expression of the RANTES chemotactic protein was observed under FF conditions at 6 and 24 hours (Figures 5G-5H).
DISCUSSION
Aging, loss of sex steroids, excess glucocorticoids and certain bone diseases such as osteoporosis, cause a decoupling in bone remodeling and a loss of bone quality due to the accumulation of apoptotic osteocytes that precede the recruitment of precursors osteoclasts and their differentiation to carry out the process of directed bone resorption14,15.
However, physiological levels of mechanical stimulation such as physical exercise maintains the viability of osteocytes and, furthermore, as demonstrated in this work, acts on their behavior by modifying the production of certain chemokines and regulating the migration of different cell types.
In our results, we found that the exposure of MC3T3-E1 pre-osteoblastic cells and human pre-adipose mesenchymal cells to conditioned media of mechanically stimulated mouse MLO-Y4 osteocitic cells does not affect their proliferation, but increases their migratory capacity. Previous studies have already shown that the conditioned medium of mechanically stimulated osteocytes is able to recruit osteoprogenitors (mesenchymal cells and osteoblasts) and promote the commitment of the osteogenic lineage of these cells to replenish depleted osteoblasts, improve bone formation and strengthen tissue16,17.
On the other hand, our results indicate a decrease in the migration of RAW 264.7 macrophages in the presence of conditioned media of MLO-Y4 cells collected after 24 hours of performing the Fluid Flow. This corroborates what has been observed by other authors who indicate that this conditioned medium is also capable of inhibiting osteoclastogenesis18.
All this suggests a negative feedback mechanism mediated by paracrine factors that would regulate the bone formation and resorption process. Therefore, we check whether certain selected chemokines are involved in this process through Western Blot assays of both the mechanically stimulated osteocytic cells and the conditio- ned media. According to our results, although there is a significant decrease in the monocyte chemotactic protein type 1 (MCP-1), the high mobility protein group 1 (HMGB1) and the RANTES chemotactic protein in the cell lysates of MLO- Y4, mechanically stimulated, do not seem to be directly associated with the migration of bone forming and repopulating cells.
However, contrary to what occurs in our study, there are previous studies that indicate that HMGB1 is released in the extracellular environment through the active secretion of stimulated cells19 and promotes osteogenic migration and differentiation of MSCs9,20. In the case of MCP-1, it has been observed that mesenchymal stem cells from the bone marrow migrate in response to this chemokine21. And there are findings that indicate that RANTES is capable of causing the migration of different cell types, including mesenchymal stem cells from bone marrow, through the induction of autophagy22-24.
For future research, carrying out a proteomic study of the osteocyte conditioned media would be required, both without stimulation and with mechanical stimulation, to deepen the communication processes of the osteocytes with their environment.
REFERENCES
1 Aguirre JI, Plotkin LI, Stewart SA, Weinstein RS, Parfitt AM, Manolagas SC, et al. Osteocyte apoptosis is induced by weightlessness in mice and precedes osteoclast recruitment and bone loss. J Bone Miner Res. 2006;21(4):605-15. [ Links ]
2 Hoey DA, Kelly DJ, Jacobs CR. A role for the primary cilium in paracrine signaling between mechanically stimulated osteocytes and mesenchymal stem cells. Biochem Biophys Res Commun. 2011;412(1):182-7. [ Links ]
3 Klein-Nulend J, Bakker AD, Bacabac RG, Vatsa A, Weinbaum S. Mechanosensation and transduction in osteocytes. Bone. 2013;54(2):182-90. [ Links ]
4 de Castro LF, Maycas M, Bravo B, Esbrit P, Gortazar A. VEGF Receptor 2 (VEGFR2)Activation Is Essential for Osteocyte Survival Induced by Mechanotransduction. J Cell Physiol. 2015;230(2):278-85. [ Links ]
5 Robinson JA, Chatterjee-Kishore M, Yaworsky PJ, Cullen DM, Zhao W, Li C, et al. Wnt/beta-catenin signaling is a normal physiological response to mechanical loading in bone. J Biol Chem. 2006;281(42):31720-8. [ Links ]
6 Yavropoulou MP, Yovos JG. The molecular basis of bone mechanotransduction. J Musculoskelet Neuronal Interact. 2016;16(3):221-36. [ Links ]
7 Bellido T. Osteocyte-driven bone remodeling. Calcif Tissue Int. 2014;94(1):25-34. [ Links ]
8 Gortazar AR, Martin-Millan M, Bravo B, Plotkin LI, Bellido T. Crosstalk between caveolin-1/extracellular signalregulated kinase (ERK) and β-cateninsurvival pathways in osteocyte mechanotransduction. J Biol Chem. 2013;288(12):8168-75. [ Links ]
9 Feng L, Xue D, Chen E, Zhang W, Gao X, Yu J, et al. HMGB1 promotes the secretion of multiple cytokines and potentiates the osteogenic differentiation of mesenchymal stem cells through the Ras/MAPK signaling pathway. Exp Ther Med. 2016;12(6):3941-7. [ Links ]
10 Zhao H, Chen D, Cao R, Wang S, Yu D, Liu Y, et al. Alcohol consumption promotes colorectal carcinoma metastasis via a CCL5-induced and AMPK pathway-mediated activation of autophagy. Sci Rep. 2018;8(1):8640. [ Links ]
11 Michael Delaine-Smith R, Javaheri B, Helen Edwards J, Vazquez M, Rumney RM. Preclinical models for in vitro mechanical loading of bone-derived cells. Bonekey Rep. 2015;19;4:728. [ Links ]
12 Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineagecells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7(2):211-28. [ Links ]
13 Kato Y, Windle JJ, Koop BA, Mundy GR, Bonewald LF. Establishment of an osteocyte-like cell line, MLO-Y4. J Bone Miner Res. 1997;12(12):2014-23. [ Links ]
14 Lee K, Kim H, Kim JM, Kim JR, Kim KJ, Kim YJ, et al. Systemic transplantationof human adipose-derived stem cells stimulates bone repair by promoting osteoblast and osteoclast function. J Cell Mol Med. 2011;15(10):2082-94. [ Links ]
15 Kogianni G, Mann V, Noble BS. Apoptotic bodies convey activity capable of initiating osteoclastogenesis and localized bone destruction. J Bone Miner Res. 2008;23(6):915-27. [ Links ]
16 Brady RT, O'Brien FJ, Hoey DA. Mechanically stimulated bone cells secrete paracrine factors that regulate osteoprogenitor recruitment, proliferation,and differentiation. Biochem Biophys Res Commun. 2015;459(1):118-23. [ Links ]
17 Turner CH, Owan I, Alvey T, Hulman J, Hock JM. Recruitment and proliferative responses of osteoblasts after mechanical loading in vivo determined using sustained-release bromodeoxyuridine. Bone. 1998;22(5):463-9. [ Links ]
18 Suzuki N, Yoshimura Y, Deyama Y, Suzuki K, Kitagawa Y. Mechanical stress directly suppresses osteoclast differentiation in RAW264.7 cells. Int J Mol Med. 2008;21(3):291-6. [ Links ]
19 Naglova H, Bucova M. HMGB1 and its physiological and pathological roles. Bratisl Lek Listy. 2012;113(3):163-71. [ Links ]
20 Xue D, Zhang W, Chen E, Gao X, Liu L, Ye C, et al. Local delivery of HMGB1 in gelatin sponge scaffolds combined with mesenchymal stem cell sheets to accelerate fracture healing. Oncotarget. 2017;8(26):42098-115. [ Links ]
21 Ryan CM, Brown JA, Bourke E, Prendergast ÁM, Kavanagh C, Liu Z, et al. ROCK activity and the Gβγ complex mediate chemotactic migration of mouse bone marrow-derived stromal cells. Stem Cell Res Ther. 2015;6:136. [ Links ]
22 Lechner J, von Baehr V. Chemokine RANTES/CCL5 as an unknown link between wound healing in the jawbone and systemic disease: is prediction and tailored treatments in the horizon? EPMA J. 2015;6(1):10. [ Links ]
23 Lu L, Zhang X, Zhang M, Zhang H, Liao L, Yang T, et al. RANTES and SDF?1 AreKeys in Cell?based Therapy of TMJ Osteoarthritis. J Dent Res. 2015;94(11): 1601-9. [ Links ]
24 Wright LM, Maloney W, Yu X, Kindle L, Collin-Osdoby P, Osdoby P. Stromal cellderived factor-1 binding to its chemokine receptor CXCR4 on precursor cells promotes the chemotactic recruitment, development and survival of human osteoclasts. Bone. 2005;36(5):840-53. [ Links ]
Received: July 10, 2018; Accepted: November 26, 2018











 texto em
texto em 


