My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista de Osteoporosis y Metabolismo Mineral
On-line version ISSN 2173-2345Print version ISSN 1889-836X
Rev Osteoporos Metab Miner vol.11 n.2 Madrid Apr./Jun. 2019 Epub Jan 20, 2020
https://dx.doi.org/10.4321/s1889-836x2019000200004
ORIGINALS
Qualitative and quantitative status of general bone in osteonecrosis of the jaws. Effect of bisphosphonates
1Universidad de Las Palmas de Gran Canaria - Instituto Universitario de Investigaciones Biomédicas y Sanitarias - Grupo de investigación en osteoporosis y metabolismo mineral - Las Palmas de Gran Canaria (España)
2Universidad de Las Palmas de Gran Canaria - Departamento de Matemáticas - Las Palmas de Gran Canaria (España)
3Departamento de Medicina - Universidad de Sevilla - Sevilla (España)
4Hospital Universitario Insular - Servicio de Cirugía Máxilofacial - Las Palmas de Gran Canaria (España)
5Hospital Universitario Insular - Unidad Metabólica Ósea - Las Palmas de Gran Canaria (España)
Objetive:
Osteonecrosis of the jaw (ONJ) is a recently reported disease whose origin and development are unknown, although prolonged bisphosphonate treatment has been attributed, among other causes. While ONJ is a localized condition, the action of bisphosphonates is widespread and affects all bones. No studies show the general bone status of patients with ONJ. Our study examines the general condition in patients with ONJ using quantitative measurements and qualitative estimates of bone by means of bone mineral density (BMD) and trabecular bone score (TBS) and ultrasound parameters in the calcaneus (QUS), along with other diseases and the taking of drugs (especially bisphosphonates) in patients with ONJ who may be involved in the pathogenesis.
Material and method:
Observational and cross-sectional study of cases and controls, conducted in 304 patients of both sexes, in which the case group (group I) was formed by 24 patients who had suffered ONJ. The control group (group II) contained 280 patients who did not present ONJ and who received bisphosphonates over at least 5 years for various reasons. All of them underwent bone densitometry (DXA, Hologic 4500 Discovery®) in the lumbar spine and proximal femur. In addition, TBS measurements were made in the lumbar spine, as well as ultrasound parameters in the calcaneus (Hologic, Sahara®) in the dominant foot (QUS).
Results:
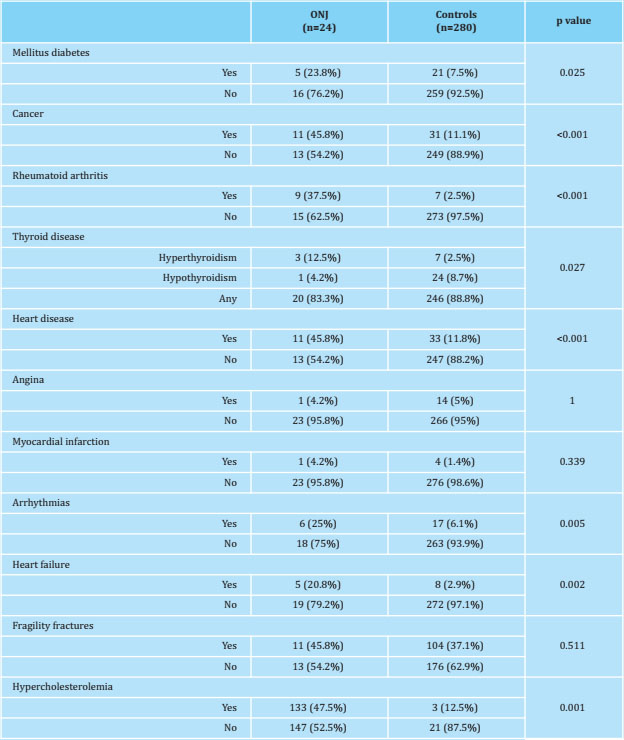
Patients suffering ONJ presented greater comorbidity than controls, with a higher prevalence of diabetes mellitus, cancer, rheumatoid arthritis, hyperthyroidism, heart disease, arrhythmias, heart failure and hypercholesterolemia. Therefore, the consumption of corticosteroids, (oral and inhaled), anticoagulants, hypnotics, bisphosphonates i.v. (zoledronate), and antineoplastic chemotherapy was also higher among patients with ONJ than control patients. However, among the patients with ONJ the percentage taking oral bisphosphonates was lower. Densitometric values (BMD measured in lumbar spine L2-L4, femoral neck and total hip) were higher in patients with ONJ compared to those in controls. The TBS showed no statistically significant differences between the two groups, and the ultrasound showed higher values of QUI and SOS in patients with ONJ than in controls. The prevalence of fragility fractures was similar in both groups.
Conclusions:
Patients with ONJ in our study presented greater comorbidity and a higher consumption of drugs than the patients in the control group, except for oral bisphosphonates. On the other hand, both BMD and ultrasound showed higher values in patients with ONJ than in controls. If we consider DXA as a technique for measuring the amount of bone mass, and TBS and calcaneal ultrasound estimating qualitative aspects of bone, we could assume that neither bone quantity nor quality in general seems to be affected in ONJ, and that its etiopathogenic mechanism is probably another. Oral bisphosphonates do not appear to be among the drugs involved in ONJ’s origin and development, but the most potent and intravenously administered bisphosphonates are, although they cannot be considered independently of the underlying disease for which they are administered.
Key words osteonecrosis; jaws; bisphosphonates; quality; quantity; densitometry; ultrasound
INTRODUCTION
Osteonecrosis of the jaw (ONJ) is a disease described fairly recently. After the reported findings by Marx1, bisphosphonates were considered the etiological agent responsible for the disease, even being called osteonecrosis due to bisphosphonates2-5, which is wrong since many factors in addition to these drugs may be implicated in the etiopathogenesis of ONJ1,6,7.
One of the hypothesis about ONJ’s development would be the existence of an excess suppression of bone remodeling, which can be produced by bisphosphonates or by other potent anti-resorptives, such as denosumab, a drug that is also involved in ONJ8,9. Since these drugs act on the entire skeleton, if there is such an excess of oversupression of bone remodelling, one could expect the existence of alterations in both the amount of BMD and bone quality in other locations. Although there are many descriptions of isolated cases or series of this disease in the literature, outlining its clinical characteristics and possible association with different diseases and risk factors1,3 7,10, we have not found publications that analyze the possible quantitative alterations and/or qualitative bone in patients with ONJ.
Bone mass measurement by dual radiological absorptiometry (DXA) has been sufficiently validated and is accepted as a reliable bone quantification technique by measuring bone mineral density (BMD)11-14. However, non-invasive bone quality measurement techniques have not been as successful, due to the many aspects that the concept of bone quality encompasses. Despite this, there are currently two techniques that can estimate some aspects of bone quality. On the one hand, trabecular bone score (TBS), associated with DXA, which offers information on bone microstructure15-21 ; and on the other, quantitative ultrasound (QUS), which although it is not known exactly what bone properties it reflects, its measurements have also been related to bone microarchitecture and some mechanical parameters22-24.
Thus, our research objective has been to study the possible alterations in the amount of bone tissue in locations other than the jaws and that serve as a reference, measured as BMD by bone densitometry (dual radiological absorptiometry, DXA) in the lumbar spine and proximal femur; as well as in bone quality, estimated, on the one hand, by means of TBS and, on the other, using the parameters obtained by QUS, in a population of patients suffering from ONJ and estimating the presence of certain diseases and treatment that affect the bone. We highlight bisphosphonates as a secondary objective, which could participate in its etiopathogenesis. For this, we take as a reference a control group of patients who had received bisphosphonates for at least 5 consecutive years and who continued to take them at the time of the study.
MATERIAL AND METHOD
Inclusion criteria
We have carried out a case and controls study in which we consider “case” to patients who had suffered an ONJ and “controls” to patients without ONJ and who had received bisphosphonates for a minimum of 5 years and continued taking it today. .
We include as cases 24 patients who were diagnosed with ONJ following the criteria of the “International Task Force on Osteonecrosis of the Jaw”25. The controls were subjects without ONJ who were recruited among patients studied in the Bone Metabolic Unit of the Insular University Hospital and who had received oral or intravenous bisphosphonates (i.v.) for a minimum of 5 years and continued receiving them.
Physical examination
All participating patients underwent a complete physical examination. Their height was obtained on a height rod and weight on a scale, with the patient wearing light clothes, without shoes. The body mass index (BMI) was calculated from the formula = weight (kg)/height (m)2.
Dual radiological absorptiometry or bone densitometry (DXA)
BMD was estimated using a Hologic® QDR 4500 Discovery densitometer (Hologic, Spain). The determinations were made in the lumbar spine (L2-L4 vertebrae) and in the proximal femur (femoral neck, trochanter, intertrochanter and total femur). The computer program provided by the manufacturer allows us to separate the anatomical locations. The results were expressed in g/cm2 and T-score. The accuracy of the equipment (coefficient of variation) was 0.5% in vitro (measured with a standard phantom) and 0.9% in vivo (obtained by double measurements made in 12 patients on the same day). All determinations were made by the same operator, so there were no inter-observer variations. T-score values were calculated from the reference values that the device includes obtained for the Spanish population.
Trabecular bone score (TBS)
All TBS measurements were carried out using the TBS iNsight Software program, version 2.0.0.1 (Med-Imaps, Pessac, France). The computer program uses the image previously obtained by DXA in the same region of interest (lumbar spine, L2-L4). T-score values were calculated from the reference values obtained for the Spanish population26.
Quantitative ultrasound (QUS)
All patients underwent an ultrasound on the calcaneus of the dominant foot. For this, we use the Sahara® Clinical Sonometer ultrasound device (Hologic Inc., Bedford, Massachusetts. USA). The system consists of 2 transducers, one of which acts as an emitter and the other as an ultrasound receiver. The parameters obtained are ultrasonic broadband attenuation (BUA) and sound speed (SOS). The results obtained by both parameters, BUA and SOS are combined to obtain the so-called ultrasonic quantitative index or QUI, when applying the formula:
QUI = 0.41 X (BUA + SOS) - 571.
In all ultrasound determinations, their corresponding T-scores were calculated with the data obtained as reference values for the Spanish population27.
Diagnosis of fractures
All patients underwent an AP and lateral dorsal and lumbar spine Rx. The prevalent vertebral fractures were diagnosed by applying the semi-quantitative Genant classification of vertebral fracture28. The presence of non-vertebral fractures was documented from the clinical history obtained from the patients confirmed by hospital medical records or by means of the appropriate radiographic studies.
Statistic analysis
Continuous data were expressed as means and standard deviations when the variables followed a normal distribution, or through the medians with their interquartile ranges when the distribution was not normal. Categorical variables were expressed as frequencies and percentages. For independent data, the percentages were compared using the Chi-Square test (X2 ) or the exact Fischer test. The averages were compared using Student's t test and the medians applying Mann Whitney's U. In all cases the level of statistical significance was considered at 5% (p value<0.05).
Ethical and legal aspects
The project was approved by the Ethics and Clinical Trials Committee of the Insular University Hospital, Gran Canaria, Spain. This is an observational study in which there was no pharmacological intervention of any kind. We observed the recommendations of the World Medical Association contained in the Declaration of Helsinki29 throughout the study.
RESULTS
Table 1 shows the baseline characteristics and lifestyles of the patients included in the study. The patients were similar in age and the proportion of men and women was similar in both groups.
Table 1 Baseline characteristics and lifestyles of patients with ONJ and controls

Continuous variables are summarized as mean ± standard deviation or as medians with their interquartile intervals (IQR). Categorical variables are expressed as frequency (%). BMI: body mass index: = (weight/size2).
The patients with ONJ was shorter in height, presented a higher BMI and a lower consumption of tobacco and alcohol than controls. We do not observe statistically significant differences in coffee consumption or physical activity in leisure time.
Table 2 shows the comorbidity of patients with ONJ and controls. Patients who suffered ONJ had a greater comorbidity than controls: they showed a higher prevalence of diabetes mellitus, cancer, rheumatoid arthritis, hyperthyroidism, heart disease, arrhythmias, heart failure and hypercholesterolemia. The prevalence of fragility fractures was similar in both groups.
In table 3, we show the consumption of drugs of both patient groups. In line with the existence of greater comorbidity, patients with ONJ had a significantly higher consumption of oral cortico-steroids, oral anticoagulants and hypnotics than controls and, similarly, a greater number of them had received chemotherapy. Inhaled steroid consumption also showed a trend that was very close to reaching the level of significance (p=0.05).
In patients with ONJ, the use of bisphosphonates was mostly via i.v. (75%) and much less orally (8.3%). Furthermore, 16.7% of these patients had never taken or received bisphosphonates. Obviously, since it was an inclusion criterion, in the control group 100% had received or taken bisphosphonates, being mostly oral (92.1%).
In table 4, we present the densitometric, ultrasound parameters and TBS values. BMD showed higher values in patients with ONJ in all anatomical locations where it was determined; both in the lumbar spine and in the proximal limb of the femur, the T-score was also higher. We did not obtain statistically significant differences in the values of TBS and Broadband Ultrasound Attenuation (BUA), while patients with ONJ showed higher values of QUI and SOS than controls.
Table 4 Bone parameters related to qualitative and quantitative aspects: BMD measured in the lumbar spine and proximal limb of the femur, TBS measured in the lumbar spine and ultrasound parameters obtained in the calcaneus

The results are expressed as medians and interquartile intervals (IQR).
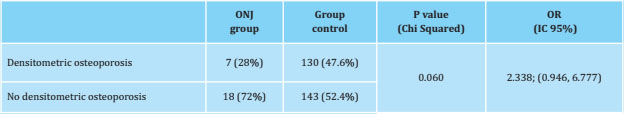
According to the densitometric values ((DXA) observed at the time of the study, we obtained that 28% of patients with ONJ had osteoporosis criteria (T-score ≤-2.5 in any of the following locations: L2-L4 , femoral neck or total hip), while these criteria were appreciated in 47.6% of the control patients, without the difference being significant (p=0.06) (Table 5).
DISCUSSION
ONJ is a relatively recent disease, the first series having been described about 15 years ago1,30-32. Its causal mechanism is unknown3,6,7,32-35 and many possible risk factors have been related, but without establishing an unequivocal cause-effect with any of them2,3,5-7,36,37, which can be considered multifactorial. For a long time, treatment with bisphosphonates has been pointed out as a primary etiologic agent of ONJ, to the point that for some time ONJ was called bisphosphonate-induced osteonecrosis1-4,31,32,35,38. This is still considered by many dental specialists who indicate their withdrawal before orodental surgery.
An etiopathogenic hypothesis that was accepted for a long time indicated that bisphosphonates, administered over several years and at high doses, would produce an excessive suppression of remodeling, which would imply bone development with a smaller amount of bone mass and a severe alteration of quality, what came to termed "frozen" bone39. In support of this hypothesis, it has been observed that the vast majority of patients with ONJ, more than 95%, are patients who have suffered from cancer and who in addition to the basic treatment of the process (surgery, radiotherapy) have received polychemotherapy and high-dose intravenous bisphosphonates, usually zoledronate1,6,7,30 at the oncological dose, which is 4 mg i.v. every 28 days (52 mg per year), while in the treatment of osteoporosis, the dose used of the same drug is 5 mg i.v. once a year40.
However, there are also some disagreements. First, a considerable percentage of patients with ONJ, 16.7%, had never received bisphosphonates.
On the other hand, the greater BMD measured by DXA in all the locations of these patients with respect to the control patients points to a greater general bone quantity of the former versus the latter. We have not found in the main databases similar studies to ours comparing BMD in patients with ONJ with controls in treatment with bisphosphonates. So we do not know whether or not this finding has been corroborated by other authors. We want to highlight the fact that there was no statistically significant difference in the densitometric diagnosis of osteoporosis between both groups. It may seem logical that there should be a higher percentage of osteoporosis diagnosis among the control group, since treatment with oral bisphosphonates (drug of choice for osteoporosis) was the majority. However, we must bear in mind that long-term treatment with this drug increases BMD, and therefore the T-score, causing its values to depart from the densitometric criteria of osteoporosis.
We also wanted to consider bone quality, a much more controversial aspect, since there is no single definitive and non-invasive method that has been accepted as the “gold standard” for estimating bone quality, unlike what happens with densitometry, which is the universally accepted reference for quantity11-14. One of the recently described methods for estimating bone quality is the socalled trabecular bone score or TBS16, which basically evaluates the integrity of the vertebral bone trabeculae, reanalyzing the DXA images17-21. The parameters obtained with QUS have also been proposed as possible indicators of bone quality22-24. In our series, patients with ONJ showed similar values of TBS and BUA to those of control patients, and the SOS and QUI rates were slightly but significantly higher in the former. This leads us to believe the qualitative bone aspects in both groups were similar, and, if anything, never worse in patients with ONJ than in those taking bisphosphonates. Some authors have described low values of ultrasound parameters in patients with ONJ41, but as with densitometry in ONJ, there are very few studies similar to ours with which to make comparisons. If we take into account that the control patients took bisphosphonates for a long time and considering that bisphosphonates improve bone quantity and quality42,43, the higher values of BMD, TBS, SOS and QUI in patients with ONJ could indicate that the general bone health of these patients is adequate.
Finally, if we add to the previous results the fact that the prevalence of fragility fractures was also similar in both groups (and considering that bisphosphonates decrease the risk of fracture), we have indirect evidence that the general bone structure, both quantitatively and qualitatively, it is the less similar (if not better) in patients with ONJ and patients under treatment with bisphosphonates.
We totally agree that the cause and development of ONJ is multifactorial, as has been published in multiple studies and agreed by consensus1-3,7,10,36. As we have observed in our series, patients with this disease have greater comorbidity. Therefore, drug use is also significantly higher in this group of patients44 than among those studied here: oral corticosteroids, inhaled corticosteroids, oral anticoagulants, hypnotics and having received polychemotherapy. However, if we focus on bisphosphonates, patients with ONJ have a greater use of potent bisphosphonates via the i.v. (75%), which was closely related to the higher incidence of cancer, but not oral bisphosphonates.
One of the limitations of this study is the sample size of the cases, with only 24 patients. This is due to the low incidence of this disease and the difficulty of getting participation in a study of these characteristics of some patients, due to its delicate clinical situation. On the other hand, the choice of the control group could be discussed. We have chosen patients who had been receiving bisphosphonates for at least 5 years, given that it is precisely this fact that is considered a risk factor associated with the appearance of ONJ45-47, but which have a beneficial effect on bone in general. Finally, we are aware that unvalued local circumstances, such as oral hygiene, or the presence of dentogingival diseases or dental interventions, have a relevant and decisive specific weight in ONJ pathogenesis, although we do not include them because they are localized circumstances that do not affect the bone in general.
CONCLUSION
Our study results indicate that patients who have suffered ONJ do not appear to have worse bone health (in terms of quantity and quality) in general compared to patients who have been receiving bisphosphonates continuously for at least 5 years. In addition, oral bisphosphonates were not the most used drugs among these patients, so we have to pay more attention to more prevalent ones such as corticosteroids, intravenous bisphosphonates, chemotherapy, hypnotics and oral anticoagulants; while taking into account comorbidities, such as cancer, diabetes, rheumatoid arthritis, hypercholesterolemia, heart disease and thyroid conditions.
Bibliografía
1 Marx RE, Sawatari Y, Fortin M, Brou-mand V. Bisphosphonate-induced exposed bone (osteonecrosis/osteopetrosis) of the jaws: Risk factors, recognition, prevention, and treatment. J Oral Ma-xillofac Surg. 2005;63(11):1567-75. [ Links ]
2 Otto S, Hafner S, Mast G, Tischer T, Volkmer E, Schieker M, et al. Bisphos-phonate-Related Osteonecrosis of the Jaw: Is pH the Missing Part in the Pathogenesis Puzzle? J Oral Maxillofac Surg. 2010;68(5):1158-61. [ Links ]
3 Allen MR, Burr DB. The Pathogenesis of Bisphosphonate-Related Osteonecrosis of the Jaw: So Many Hypotheses, So Few Data. J Oral Maxillofac Surg. 2009;67(Suppl 5):61-70. [ Links ]
4 Lehrer S, Montazem A, Ramanathan L, Pessin-Minsley M, Pfail J, Stock RG, et al. Bisphosphonate-Induced Osteonecrosis of the Jaws, Bone Markers, and a Hypothesized Candidate Gene. J Oral Maxillofac Surg. 2009;67(1):159-61. [ Links ]
5 Rasmusson L, Abtahi J. Bisphospho-nate Associated Osteonecrosis of the Jaw: An Update on Pathophysiology, Risk Factors, and Treatment. Int J Dent. 2014;2014:1-9. [ Links ]
6 Sosa Henríquez M, Vicente Barrero M, Bocanegra Pérez S. Osteonecrosis de los maxilares: nuevas evidencias sobre su etiopatogenia. Rev Osteoporos y Metab Miner. 2011;3(1)5-6. [ Links ]
7 Sosa Henríquez M, Gómez de Tejada Romero MJ, Bagán Sebastián JV, Curiel MD, Jódar Gimeno E, et al. Osteonecrosis de los maxilares: Documento de consenso. Rev Osteoporos y Metab Miner. 2009;1(1)4-51. [ Links ]
8 Selvi Sabater P, Rizo Cerdá AM, Titos Arcos JC, Espuny Miró A. Posible osteonecrosis mandibular inducida por denosumab en el tratamiento de la osteoporosis. A propósito de un caso. Farm Hosp. 2014;38(3):248-9. [ Links ]
9 Troeltzsch M, Woodlock T, Kriegelstein S, Steiner T, Messlinger K, Troeltzsch M. Physiology and pharmacology of non-bisphosphonate drugs implicated in osteonecrosis of the jaw. J Can Dent Assoc (Tor). 2012;78(1). [ Links ]
10 Leizaola-Cardesa IO, Aguilar-Salvatierra A, Gonzalez-Jaranay M, Moreu G, Sala-Romero MJ, Gómez-Moreno G. Bisphosphonates, vitamin D, parathyroid hormone, and osteonecrosis of the jaw. Could there be a missing link? Med Oral Patol Oral y Cir Bucal. 2016;21(2):e236-40. [ Links ]
11 National Institutes of Health (NIH) Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. JAMA 2001;285(6):785-95. [ Links ]
12 Sosa Henríquez M, Gómez de Tejada Romero MJ. ¿Hay vida más allá de la densitometría ósea? Med Clin (Barc). 2011;136(14):607-52. [ Links ]
13 Gómez de Tejada Romero MJ, Sosa Henríquez M. Los ultrasonidos, la densitometría, el T-score y los criterios de la organización mundial de la salud para el diagnóstico de la osteoporosis. Rev Esp Enf Metab Oseas. 2002; 11(5):165-87. [ Links ]
14 Gómez de Tejada Romero MJ, Sosa Henríquez M. Los ultrasonidos, la den-sitometría y la osteoporosis. An Med Interna. 2007;24(2):55-6. [ Links ]
15 Martineau P, Leslie WD. Trabecular bone score (TBS): Method and applications. Bone. 2017;104:66-72. [ Links ]
16 Silva BC, Boutroy S, Zhang C, McMahon DJ, Zhou B, Wang J, et al. Trabecular bone score (TBS) - A novel method to evaluate bone microarchitectural texture in patients with primary hyperparathyroidism. J Clin Endocrinol Metab. 2013;98(5):1963-70. [ Links ]
17 Pothuaud L, Barthe N, Krieg MA, Meh-sen N, Carceller P, Hans D. Evaluation of the Potential Use of Trabecular Bone Score to Complement Bone Mineral Density in the Diagnosis of Osteoporosis: A Preliminary Spine BMD-Matched, Case-Control Study. J Clin Densitom. 2009;12(2):170-6. [ Links ]
18 Bousson V, Bergot C, Sutter B, Levitz P, Cortet B. Trabecular bone score (TBS): Available knowledge, clinical relevance, and future prospects. Osteoporos Int. 2012;23(5):1489-501. [ Links ]
19 Warzecha M, Czerwiñski E, Amaro-wicz J, Berwecka M. Trabecular Bone Score (TBS) in Clinical Practice - Review. Ortop Traumatol Rehabil. 2018; 20(5):347-359. [ Links ]
20 Harvey NC, Glüer CC, Binkley N, McCloskey E V., Brandi ML, Cooper C, et al. Trabecular bone score (TBS) as a new complementary approach for osteoporosis evaluation in clinical practice. Bone. 2015;78:216-24. [ Links ]
21 Leslie WD, Krieg MA, Hans D. Clinical factors associated with trabecular bone score. J Clin Densitom [Internet]. 2013;16(3):374-9. [ Links ]
22 Raum K, Grimal Q, Varga P, Barkmann R, Glüer CC, Laugier P. Ultrasound to assess bone quality. Curr Osteoporos Rep. 2014;12(2):154-62. [ Links ]
23 Wallach S, Feinblatt JD, Carstens JH, Avioli L V. The bone “quality" problem. Calcif Tissue Int. 1992;51(3):169-72. [ Links ]
24 Glüer CC. Quantitative Ultrasound-It is time to focus research efforts. Bone. 2007;40(1):9-13. [ Links ]
25 Khan AA, Morrison A, Hanley DA, Fel-senberg D, McCauley LK, O'Ryan F, et al. Diagnosis and management of osteonecrosis of the jaw: A systematic review and international consensus. J Bone Miner Res. 2015;30:3-23. [ Links ]
26 Del Rio LM, Winzenrieth R, Cormier C, Di Gregorio S. Is bone microarchitecture status of the lumbar spine assessed by TBS related to femoral neck fracture? A Spanish case-control study. Osteoporos Int. 2013;24(3):991-8. [ Links ]
27 Sosa M, Saavedra P Muñoz-Torres M, Alegre J, Gómez C, González-Macías J, et al. Quantitative ultrasound calcaneus measurements: Normative data and precision in the Spanish population. Osteoporos Int. 2002;13(6):487-92. [ Links ]
28 Genant HK, Wu CY, van Kuijk C, Nevitt MC. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res. 1993;8(9):1137-48. [ Links ]
29 World Medical Association. World Medical Association Declaration of Helsinki. Ethical Principles for Medical Research Involving Human Subjects. JAMA. 2013;310(20):2013-6. [ Links ]
30 Raje N, Woo S Bin, Hande K, Yap JT, Richardson PG, Vallet S, et al. Clinical, radiographic, and biochemical characterization of multiple myeloma patients with osteonecrosis of the jaw. Clin Cancer Res. 2008;14(8):2387-95. [ Links ]
31 Lehrer S, Montazem A, Ramanathan L, Pessin-Minsley M, Pfail J, Stock RG, et al. Normal serum bone markers in bis-phosphonate-induced osteonecrosis of the jaws. Oral Surgery, Oral Med Oral Pathol Oral Radiol Endodontology. 2008;106(3):389-91. [ Links ]
32 Assael LA. Oral Bisphosphonates as a Cause of Bisphosphonate-Related Osteonecrosis of the Jaws: Clinical Findings, Assessment of Risks, and Preventive Strategies. J Oral Maxillofac Surg 2009;67(Suppl 5):35-43. [ Links ]
33 Aspenberg P Osteonecrosis of the jaw: what do bisphosphonates do? Expert Opin Drug Saf. 2006;5(6):743-5. [ Links ]
34 Bedogni A, Saia G, Bettini G, Tronchet A, Totola A, Bedogni G, Osteomlacia: the missing link in the pathogenesis of bis-phosphonate-related osteonecrosis of the jaws? Oncologist. 2012;17:1114-9. [ Links ]
35 Kwon YD, Ohe JY, Kim DY, Chung DJ, Park YD. Retrospective study of two biochemical markers for the risk assessment of oral bisphosphonate-related osteonecrosis of the jaws: Can they be utilized as risk markers? Clin Oral Implants Res. 2011;22(1):100-5. [ Links ]
36 Ruggiero S, Gralow J, Marx RE, Hoff AO, Schubert MM, Huryn JM, et al. Practical Guidelines for the Prevention, Diagnosis, and Treatment of Osteonecrosis of the Jaw in Patients With Cancer. J Oncol Pract. 2017;2(1):7-14. [ Links ]
37 Khan AA, Morrison A, Hanley DA, Fel-senberg D, McCauley LK, O'Ryan F, et al. Diagnosis and management of osteonecrosis of the jaw: A systematic review and international consensus. J Bone Miner Res. 2015;30(1):3-23. [ Links ]
38 Huang YF, Chang CT, Muo CH, Tsai CH, Shen YF, Wu CZ. Impact of bisphospho-nate-related osteonecrosis of the jaw on osteoporotic patients after dental extraction: A population-based cohort study. PLoS One. 2015;10(4):1-13. [ Links ]
39 Aspenberg P, Schilcher J, Fahlgren A. Histology of an undisplaced femoral fatigue fracture in association with bisphosphonate treatment: Frozen bone with remodelling at the crack. Acta Orthop. 2010;81(4):460-2. [ Links ]
40 Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley J. Once-Yearly Zo-ledronic Acid for Treatment of Postmenopausal Osteoporosis. N Engl J Med. 2007;356(18):1809-22. [ Links ]
41 Motta ACF, Macedeo LD, Santos GG, Guerreiro CT, Ferrari T, Oliveira TFL, et al. Quantitative ultrasound at the hand phalanges in patients with bisphos-phonate-related osteonecrosis of the jaws. Braz Oral Res. 2015;29(1):1-9. [ Links ]
42 Delmas PD, Li Z, Cooper C. Relationship between changes in bone mineral density and fracture risk reduction with antiresorptive drugs: some issues with meta-analyses. J Bone Miner Res. 2004;19(2):330-7. [ Links ]
43 Gallacher SJ, Dixon T. Impact of treatments for postmenopausal osteoporosis (bisphosphonates, parathyroid hormone, strontium ranelate, and de-nosumab) on bone quality: a systematic review. Calcif Tissue Int. 2010;87: 469-84. [ Links ]
44 Sosa-Henriquez M. Osteonecrosis de los maxilares: Documento de consenso. Rev Osteoporos y Metab Miner. 2009;1(1):41-51. [ Links ]
45 Ramírez L, López-Pintor RM, Casañas E, Arriba L de, Hernández G. New Non-Bisphosphonate Drugs that Produce Osteonecrosis of the Jaws. Oral Health Prev Dent. 2015;13(5):385-93. [ Links ]
46 Adler RA, Fuleihan GE, Bauer DC, Camacho PM, Bart L, Clines GA, et al. Managing Osteoporosis in Patients on Long-Term Bisphosphonate Treatment: Report of a Task Force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2016;31(1):16-35. [ Links ]
47 Anagnostis P, Paschou SA, Mintziori G, Ceausu I, Depypere H, Lambrinoudaki I, et al. Drug holidays from bisphos-phonates and denosumab in postmenopausal osteoporosis: EMAS position statement [Internet]. Vol. 101, Maturitas. 2017;101:23-30. [ Links ]
48 Black DM, Bauer DC, Schwartz A V, Cummings SR, Rosen CJ. Continuing Bisphosphonate Treatment for Osteoporosis - For Whom and for How Long? N Engl J Med. 2012;366(22): 2051-3. [ Links ]
Received: September 15, 2019; Accepted: September 30, 2019











 text in
text in