Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista de Osteoporosis y Metabolismo Mineral
versão On-line ISSN 2173-2345versão impressa ISSN 1889-836X
Rev Osteoporos Metab Miner vol.12 no.3 Madrid Jul./Set. 2020 Epub 25-Jan-2021
https://dx.doi.org/10.4321/s1889-836x2020000300003
ORIGINALS
Persistence to aromatase inhibitors in the SIDIAP cohort: mortality and influence of bisphosphonates
1 Hospital del Mar Institute for Medical Research (IMIM). Center for Biomedical Research Network on Frailty and Healthy Aging (CIBERFES). Barcelona (Spain)
2Department of Internal Medicine. Hospital del Mar. Autonomous University of Barcelona. Barcelona (Spain)
3Department of Medical Oncology. Hospital del Mar Institute for Medical Research (IMIM). Barcelona (Spain)
4Research Group on Prevalent Diseases of the Locomotor System in Primary Care (GREMPAL). Jordi Gol University Institute for Research in Primary Care (IDIAP Jordi Gol) and Center for Biomedical Research Network on Frailty and Aging Healthy (CIBERFES). Autonomous University of Barcelona and Carlos III Health Institute. Barcelona (Spain)
5 Center for Statistics in Medicine (CSM). Nuffield Department of Orthopedics, Rheumatology and Musculoskeletal Sciences (NDORMS). Oxford University. Oxford (UK)
Objetive
To assess the persistence of aromatase inhibitor (AI) therapy, mortality associated with treatment discontinuation and the influence of oral bisphosphonates (BP) in routine clinical practice.
Material and methods
Prospective observational study of women with breast cancer undergoing AI treatment between January 2006 and December 2015, registered in the SIDIAP database. Those previously treated with tamoxifen were excluded. AI persistence was studied with a survival analysis: the Kaplan-Meier estimator was calculated, and a proportional hazards model (Cox regression) was performed between users and non-users of BP adjusting for age. A sensitivity analysis was carried out taking into account mortality as a competitive risk (Fine and Gray models). The difference in mortality between groups was compared using a Chi square test.
Results
A persistence to AI of 87% was observed after 5 years of treatment, with an overall mortality of 19.75%. There was 7.7% less mortality in those patients who completed the 5 years of treatment compared to those who did not. Patients with BP showed a decrease in mortality (6.6%) and a decrease in the risk of discontinuing therapy (adjusted SHR:
0.62 [95% CI: 0.55 to 0.70]) compared to non-users.
Conclusions
Persistence to AI and BP use are associated with a decrease in overall mortality. Furthermore, the use of BP increases adherence to AI treatment.
Key words aromatase inhibitors; bisphosphonates; breast cancer; mortality; persistence
INTRODUCTION
Aromatase inhibitors (AIs) are the recommended adjuvant therapy to treat estrogen receptor-positive breast cancer1,2. Its effectiveness in reducing the risk of recurrence and mortality is acknowledged3. However, AIs are also associated with various side effects that affect patients’ quality of life and therefore compromise adherence to treatment and associated mortality4.
Reportedly, 30% of patients prescribed with AI discontinue their treatment due to adverse events5, mainly musculoskeletal6,7. Among them, the most frequent are arthralgias8 and accelerated loss of bone mass9 associated with an increase in osteoporotic fracture10,11. To prevent the loss of bone mass, treating patients with antiresorptives is recommended, with bisphosphonates (BP) being the most used12-14.
BP use has been associated with improved mortality associated with reduced bone metastases13. Similarly, a study published in a Korean population showed the use of BP was associated with improved adherence15.
Our study’s objective was to evaluate the persistence of AI therapy, the mortality associated with treatment discontinuation, and the influence of oral BPs, in a population-based cohort with data obtained from routine clinical practice.
MATERIAL AND METHODS
Data Base
Data from more than 7 million patients, coming from more than 350 Primary Care centers in Catalonia, are registered anonymously by the Information System for the Development of Research in Primary Care (SIDIAP), covering >80% of the total of the Catalan population (http://www.sidiap.org).
This database contains information on sociodemographic variables, lifestyle risk factors (alcohol consumption, obesity, smoking, etc.), comorbidities, and pharmacological dispensations. The data are collected by professionals in the health sector, including the codes of the international classification of diseases and related health problems, 10th edition (ICD-10), as well as structured forms for the collection of clinical variables (tobacco, index of body mass, etc.). SIDIAP also has registered mortality data, obtained from the Central Registry of Insured Persons, as well as migration outside the catchment area16.
Study design and participants
Prospective observational study of women diagnosed with hormone receptor-positive breast cancer undergoing AI treatment. Patients treated with AI in monotherapy between January 2006 and December 2015 collected in the SIDIAP database were included. AI users were identified using the ATC (European Pharmaceutical Substances and Medicines Coding System) codes: L02BG03 for anastrozole, L02BG04 for letrozole, and L02BG06 for exemestane. Those with a previous history of cancer (except local non-melanoma skin cancer) were excluded.
Patients’ follow-up period
For the adherence study, patients were monitored from the start of AI therapy until the first of the following events: cessation or abandonment of AI therapy, death, migration out of the catchment area, or end of availability of data in SIDIAP (December 31, 2015). In the case of mortality, the patients were followed up from their entry into the study until December 31, 2015.
Study variables
The main study variables were adherence to AI, and overall survival. The continued use of AIs was studied through pharmaceutical billing records. Treatment cessation or abandonment was considered in those records without dispensing with intervals of 6 months or more. Overall survival, expressed in mortality, was reported during the follow-up period.
The effect of BPs on persistence and mortality was studied by stratifying in users and non-users: patients with oral BP records (M05BA) were classified as BP users with codes M05BA01 (etidronic acid), M05BA02 (clodronic acid ), M05BA04 (alendronic acid), M05BA05 (tiludronic acid), M05BA06 (ibandronic acid), M05BA07 (risedronic acid), and M05BB03 (combination of alendronic acid and cholecalciferol).
Statistical Analysis
Patient characteristics were described using the mean ± standard deviation (SD) in the quantitative variables with normal distribution, and the number and percentage –n (%)– for the categorical variables.
Adherence to AI treatment was studied with a survival analysis: the Kaplan-Meier estimator was calculated and represented by cumulative probability models. A proportional hazards model (Cox regression) was carried out between users and non-users of BP adjusting for age, obtaining the hazard ratio (HR), and its proportionality verified. Additionally, a sensitivity analysis took into account mortality as a competitive risk (Fine and Gray models), estimating the sub-distribution of the risk ratios (SHR).
Finally, the difference in mortality between groups was compared using a Chi square test.
The analyzes were carried out with R 3.5.3 using the foreign, compare groups, splines, survival, and survminer packages. These were defined as significant with p<0.05.
RESULTS
18,455 data were collected from women treated with AI. Its baseline characteristics are described in table 1. The persistence [95% CI] to AI treatment was 99.8% [99.7 to 99.9] at 1 year, 98.3% [98.1 to 98 , 5] at 2 years, 95.8% [95.5 to 96.2] at 3 years, 92.9% [92.4 to 93.4] at 4 years, and 87.0% [86.3 to 87.8] after 5 years of treatment (Figure 1).
Table 1. Baseline characteristics of the participants included in the study
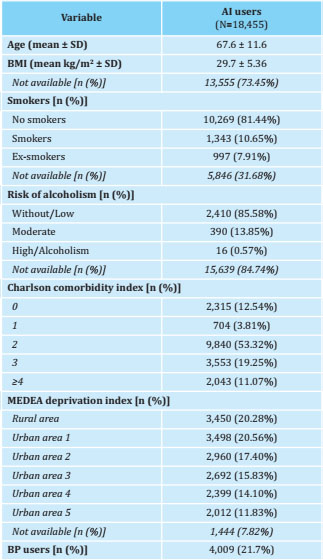
BP: bisphosphonates; SD: standard deviation; BMI: body mass index; MEDEA: mortality in small Spanish areas and socio-economic and environmental inequalities.
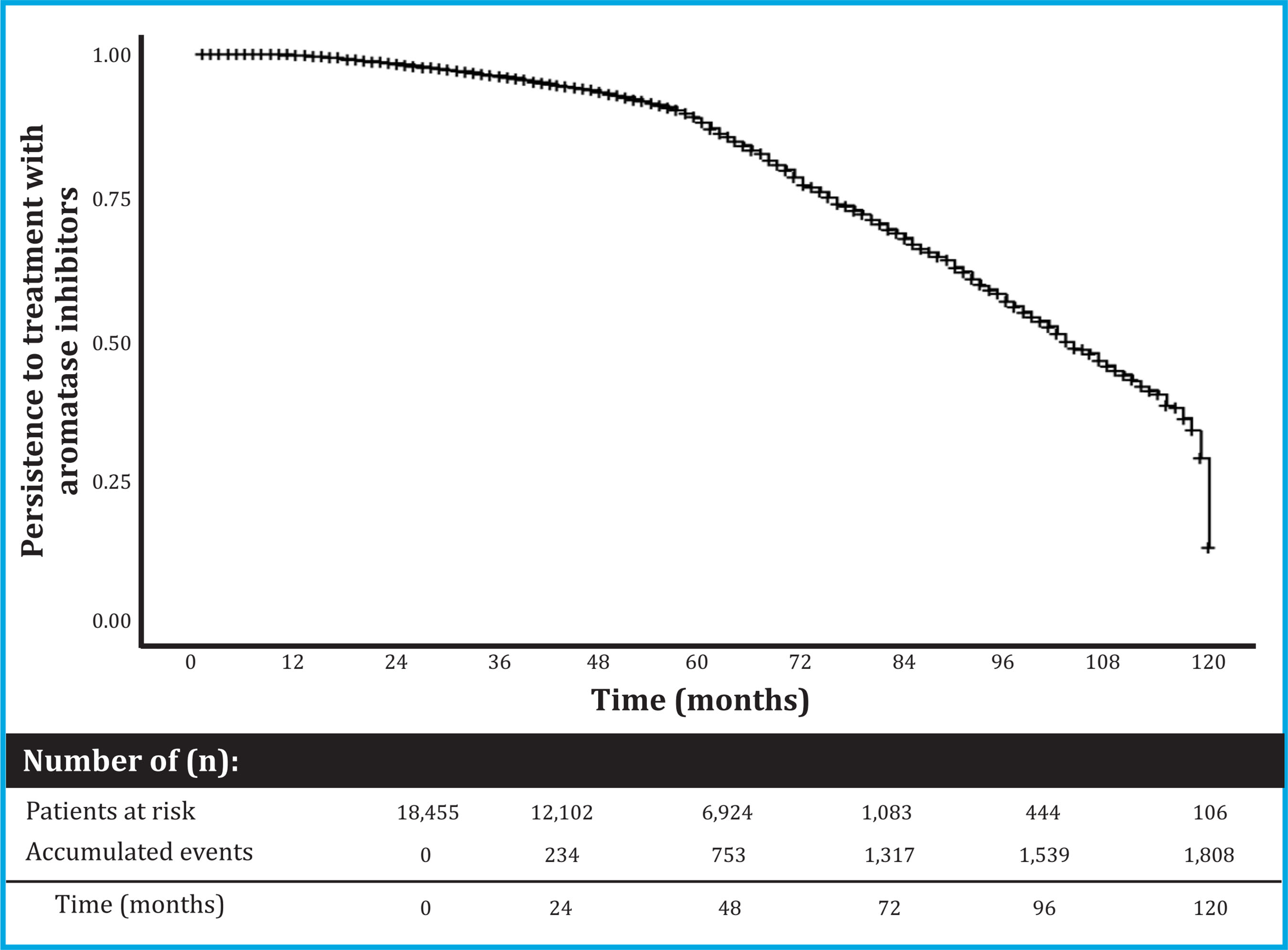
Figure 1. Persistence to AI treatment. The graph presents a Kaplan-Meyer curve that shows the risk of AI abandonment in cumulative terms. Abbreviations: AI: aromatase inhibitors
Mortality was quantified by stratifying the patients taking into account those who completed 5 years of treatment, and, on the other hand, those who did not. An overall mortality of 19.75% (3,644/18,455) was observed: with 21.2% (3,165/14,908) in patients who did not complete 5 years of AI treatment, and 13.5% (479/3,547) in those treated for 5 years or more (p<0.001).
Influence of the BP
Of the 18,455 patients included in the study, 21.7% (n=4,009) were treated with oral BP (Table 2). They showed better persistence to AI than those not treated with BP: 99.9% [99.8 to 100] vs. 99.7% [99.6 to 99.8] at 1 year; 99.8% [99.6 to 99.9] vs. 97.8% [97.5 to 98.1] at 2 years; 98.5% [98.1 to 98.9] vs. 94.9% [94.4 to 95.3] at 3 years; 97.2% [96.6 to 97.8] vs. 91.2% [90.5 to 91.8] at 4 years; and 93.3% [92.2 to 94.4] vs. 84.5% [83.5 to 85.5] at 5 years of treatment, respectively (Figure 2). In this way, the risk ratio of abandoning AIs in BP users compared to non-users was as follows: adjusted HR: 0.53 [95% CI: 0.47 to 0.60], and adjusted SHR: 0, 62 [95% CI: 0.55-0.70].
Table 2. Baseline characteristics of women treated with AI according to their use of BP
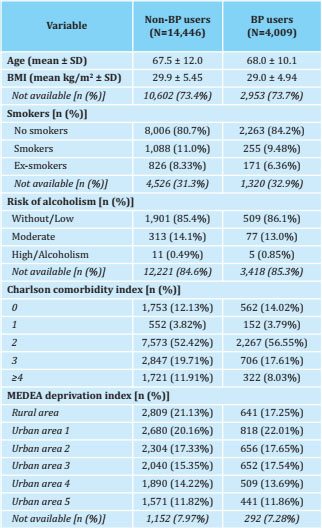
BP: bisphosphonates; SD: standard deviation; BMI: body mass index; MEDEA: mortality in small Spanish areas and socio-economic and environmental inequalities.
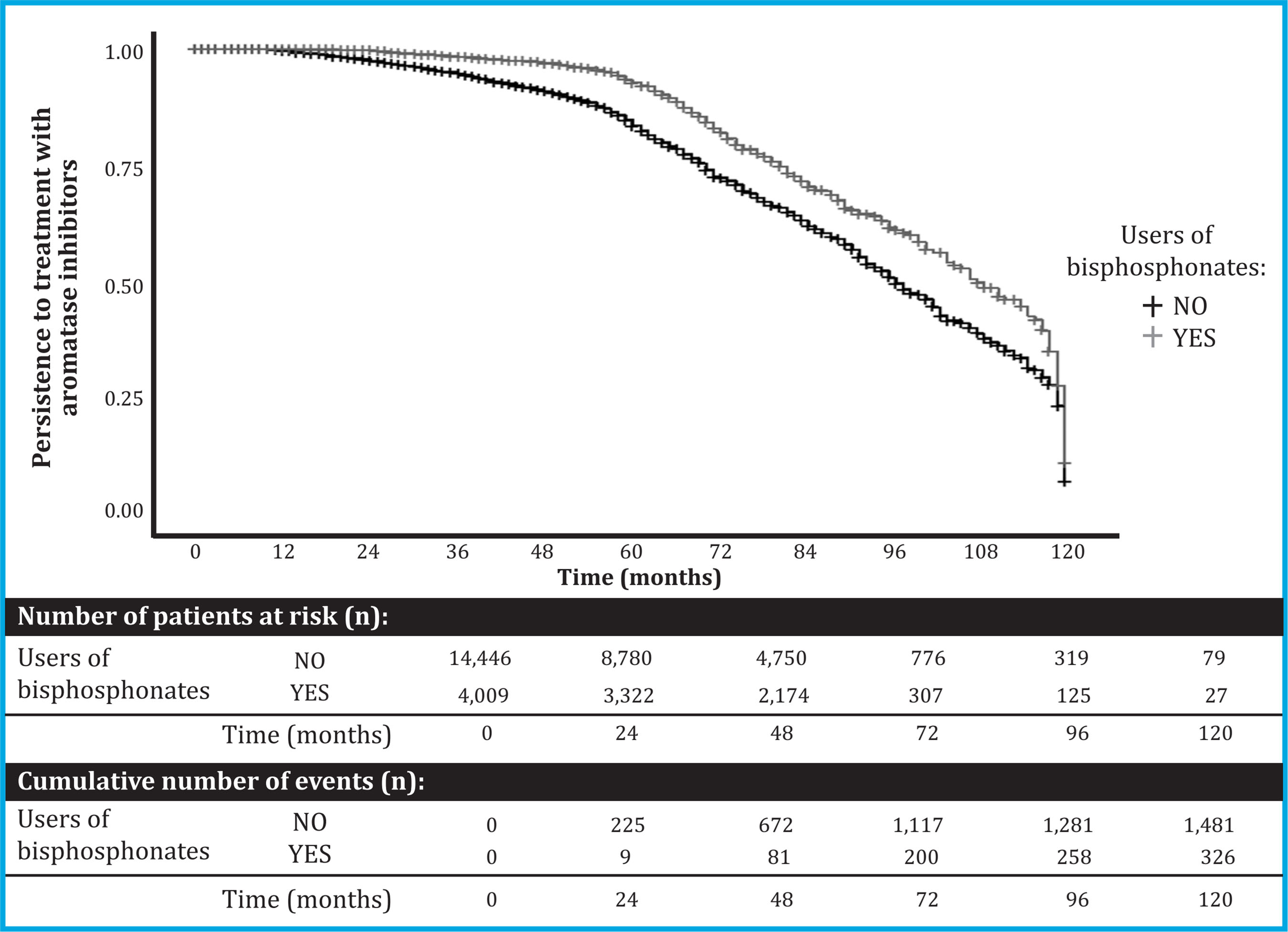
Figure 2. Persistence of AI treatment among users and nonusers of BP. The graph presents a Kaplan-Meyer curve that shows AI drop out risk in cumulative terms between the study groups: users and non-users of BP. Abbreviations: AI: aromatase inhibitors; BP: bisphosphonates
In contrast, mortality in patients with BP was 14.6% (587/4,009), while in non-users it was 21.2% (3,507/ 14,446) (p<0.001).
DISCUSSION
This study evaluates the persistence of AI therapy in a cohort of women diagnosed with hormone receptor-positive breast cancer, as well as mortality and the effect of bisphosphonates, in routine clinical practice. It was observed that the global persistence at 5 years was 87% with an overall mortality of 19.75%. Mortality in those patients who completed 5 years of therapy was 7.7% lower than those who did not. On the other hand, FB users showed better persistence to AI treatment, with a 47% lower risk of discontinuing therapy, and 6.6% lower mortality than non-users.
Reports indicate the side effects of AI negatively influence adherence to treatment5. Several randomized controlled trials (RCTs) have published persistence rates that vary between 76-90%17,18. However, the reliability of these percentages may be questioned by the lack of discontinuity results from some RCTs. Several studies of adherence in population databases show values of around 69-88% in short observation periods (one year of adherence)19-21, and of 61-79% in longer follow-up periods (3-4,5 years)20,22. Hershman et al. (2010)22 observed a 30% discontinuation rate in patients with AI at 4.5 years of follow-up, while Hadji et al. (2013)23 described a discontinuation between 44-55% at 3 years. Among other factors, the age of the patients (younger, less adherence), and the cost of medicines and/or derived medical expenses –especially in private health systems–, have been described as variables associated with greater discontinuity21. The high persistence observed in our population could be explained by a high mean age (mean ± SD: 67.6 ± 11.6) compared to that reported in the RCTs (mean ± SD: 64.1 ± 9.0 in the study ATAC24, and 64.3 ± 8.1 in the IES study25; median [range]: 61 [38-89] in the BIG 1-98 study26; and median of 63.9 and 64.3 in patients with exemestane and anastrozole in study MA.2727) and a public health system, where the cost of treatment is practically negligible.
In the case of global mortality, there is a certain diversity of results depending on the design of the RCT. In the BIG 1-98 study, a mortality of 12.3% was observed28. The ATAC safety study detected a mortality of 23.5% in all AI monotherapy users, and 21.5% in the subpopulation of women with known hormone receptor-positive tumor status29. In contrast, study MA.27, published by Goss et al. (2013), showed a mortality of 5.7% at 5 years27. Unlike RCTs, our study uses data from the general population visited in primary care, achieving a more representative population of the usual clinic. Interestingly, the mortality values of our study population are similar to those described in the ATAC study. This fact could be attributed to the fact that both report mortality results of up to 10 years of follow-up.
On the other hand, and in agreement with our results, the use of BP was reported by Lee et al. (2014)15 as a factor that improves adherence to AI treatment. Likewise, the use of BP was associated with a 34% decrease in the incidence of bone metastases and a 17% reduction in mortality13. In general, the use of BP decreases overall mortality, increases life expectancy and prevents the appearance of various cancers in the general population30. This improvement in life expectancy is not only attributed to the decrease in fractures31, but also to a possible prevention of frailty and a greater capacity of the individual to cope with different conditions32.
Taking all this into account, the greater adherence to AI in patients treated with BP could be explained by improved treatment of adverse events that would have a positive impact on the patient, while the decrease in overall mortality derived from the use of the BP could be attributed both to a decrease in bone metastases and to greater adherence to AIs.
One limitation of this study is that the SIDIAP database does not have data referring to the cause of mortality or the reason for discontinuation of treatment. Thus, our study only considers overall mortality, but there is a risk of bias in that mortality before 5 years is not a consequence of discontinuing therapy. Additional studies are needed to verify that the observed difference in mortality is not due to a bias in the populations studied (between those patients who completed 5 years vs. those who did not, and between users and nonusers of BP). However, our study corroborates the results observed in previous studies.
In conclusion, a 5-year persistence to AI of 87% has been observed in routine clinical practice, which improves with the use of BP. On the other hand, completing 5 years of AI therapy and the use of BP would be associated with a decrease in mortality.
Funding: This work was funded by the FEIOMM Mobility Grant 2018, the Center for Biomedical Research on Frailty and Healthy Aging Network (CIBERFES; CB16/10/00245), the FIS (PI16/ 00818) of the ISCIII and FEDER funds.
Ethics statement: This study was approved by the IDIAP Jordi Gol Research Ethics Committee (CEI) and the SIDIAP Scientific Committee (P16/031). The data from the SIDIAP database were anonymized, with a null identification risk, in accordance with Organic Law 15/1999, of December 13. Therefore, the signing of an informed consent by the patients was not required.
REFERENCES
1 Cardoso F, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rubio IT, et al. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30(8):1194-220. [ Links ]
2 Liedtke C, Jackisch C, Thill M, Thomssen C, Muller V, Janni W, et al. AGO Recommendations for the diagnosis and treatment of patients with early breast cancer: Update 2018. Breast Care (Basel). 2018;13(3):196-208. [ Links ]
3 Early Breast Cancer Trialists' Collaborative G. Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet. 2015;386(10001):1341-52. [ Links ]
4 Ryden L, Heibert Arnlind M, Vitols S, Hoistad M, Ahlgren J. Aromatase inhibitors alone or sequentially combined with tamoxifen in postmenopausal early breast cancer compared with tamoxifen or placebo - Meta-analyses on efficacy and adverse events based on randomized clinical trials. Breast. 2016;26:106-14. [ Links ]
5 Kadakia KC, Snyder CF, Kidwell KM, Seewald NJ, Flockhart DA, Skaar TC, et al. Patient-reported outcomes and early discontinuation in aromatase inhibitor-treated postmenopausal women with early stage breast cancer. Oncologist. 2016;21(5):539-46. [ Links ]
6 Henry NL, Azzouz F, Desta Z, Li L, Nguyen AT, Lemler S, et al. Predictors of aromatase inhibitor discontinuation as a result of treatment-emergent symptoms in early-stage breast cancer. J Clin Oncol. 2012;30(9):936-42. [ Links ]
7 Pineda-Moncusi M, Servitja S, Tusquets I, Diez-Perez A, Rial A, Cos ML, et al. Assessment of early therapy discontinuation and health-related quality of life in breast cancer patients treated with aromatase inhibitors: BABLE cohort study. Breast Cancer Res Treat. 2019;177(1):53-60. [ Links ]
8 Niravath P. Aromatase inhibitor-induced arthralgia: a review. Ann Oncol. 2013;24(6):1443-9. [ Links ]
9 Pineda-Moncusi M, Servitja S, Casamayor G, Cos ML, Rial A, Rodriguez-Morera J, et al. Bone health evaluation one year after aromatase inhibitors completion. Bone. 2018;117:54-9. [ Links ]
10 Goldvaser H, Barnes TA, Seruga B, Cescon DW, Ocana A, Ribnikar D, et al. Toxicity of extended adjuvant therapy with aromatase inhibitors in early breast cancer: A Systematic review and meta-analysis. J Natl Cancer Inst. 2018;110(1):31-9. [ Links ]
11 Pineda-Moncusi M, Garcia-Giralt N, Diez-Perez A, Servitja S, Tusquets I, PrietoAlhambra D, et al. Increased fracture risk in women treated with aromatase inhibitors versus tamoxifen: beneficial effect of bisphosphonates. J Bone Miner Res. 2019;35(2):291-7. [ Links ]
12 Hadji P, Coleman RE, Wilson C, Powles TJ, Clezardin P, Aapro M, et al. Adjuvant bisphosphonates in early breast cancer: consensus guidance for clinical practice from a European Panel. Ann Oncol. 2016;27(3):379-90. [ Links ]
13 Hadji P, Aapro MS, Body JJ, Gnant M, Brandi ML, Reginster JY, et al. Management of Aromatase Inhibitor-Associated Bone Loss (AIBL) in postmenopausal women with hormone sensitive breast cancer: Joint position statement of the IOF, CABS, ECTS, IEG, ESCEO IMS, and SIOG. J Bone Oncol. 2017;7:1-12. [ Links ]
14 Tremollieres FA, Ceausu I, Depypere H, Lambrinoudaki I, Mueck A, Perez-Lopez FR, et al. Osteoporosis management in patients with breast cancer: EMAS position statement. Maturitas. 2017;95:65-71. [ Links ]
15 Lee HS, Lee JY, Ah YM, Kim HS, Im SA, Noh DY, et al. Low adherence to upfront and extended adjuvant letrozole therapy among early breast cancer patients in a clinical practice setting. Oncology. 2014;86(5-6):340-9. [ Links ]
16 Bolibar B, Fina Aviles F, Morros R, Garcia-Gil M del M, Hermosilla E, Ramos R, et al. Base de datos SIDIAP: la historia clinica informatizada de Atencion Primaria como fuente de informacion para la investigacion epidemiologica. Med Clin (Barc). 2012;138(14):617-21. [ Links ]
17 Verma S, Madarnas Y, Sehdev S, Martin G, Bajcar J. Patient adherence to aromatase inhibitor treatment in the adjuvant setting. Curr Oncol. 2011;18 Suppl 1:S3-9. [ Links ]
18 Chlebowski RT, Geller ML. Adherence to endocrine therapy for breast cancer. Oncology. 2006;71(1-2):1-9. [ Links ]
19 Ziller V, Kalder M, Albert US, Holzhauer W, Ziller M, Wagner U, et al. Adherence to adjuvant endocrine therapy in postmenopausal women with breast cancer. Ann Oncol. 2009;20(3):431-6. [ Links ]
20 Partridge AH, LaFountain A, Mayer E, Taylor BS, Winer E, Asnis-Alibozek A. Adherence to initial adjuvant anastrozole therapy among women with early-stage breast cancer. J Clin Oncol. 2008;26(4):556-62. [ Links ]
21 Sedjo RL, Devine S. Predictors of non-adherence to aromatase inhibitors among commercially insured women with breast cancer. Breast Cancer Res Treat. 2011;125(1):191-200. [ Links ]
22 Hershman DL, Kushi LH, Shao T, Buono D, Kershenbaum A, Tsai WY, et al. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol. 2010;28(27):4120-8. [ Links ]
23 Hadji P, Ziller V, Kyvernitakis J, Bauer M, Haas G, Schmidt N, et al. Persistence in patients with breast cancer treated with tamoxifen or aromatase inhibitors: a retrospective database analysis. Breast Cancer Res Treat. 2013;138(1):185-91. [ Links ]
24 Baum M, Budzar AU, Cuzick J, Forbes J, Houghton JH, Klijn JG, et al. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet. 2002;359(9324):2131-9. [ Links ]
25 Coombes RC, Hall E, Gibson LJ, Paridaens R, Jassem J, Delozier T, et al. A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med. 2004;350(11):1081-92. [ Links ]
26 Thurlimann B, Keshaviah A, Coates AS, Mouridsen H, Mauriac L, Forbes JF, et al. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med. 2005;353(26):2747-57. [ Links ]
27 Goss PE, Ingle JN, Pritchard KI, Ellis MJ, Sledge GW, Budd GT, et al. Exemestane versus anastrozole in postmenopausal women with early breast cancer: NCIC CTG MA.27-a randomized controlled phase III trial. J Clin Oncol. 2013;31 (11):1398-404. [ Links ]
28 Mouridsen H, Giobbie-Hurder A, Goldhirsch A, Thurlimann B, Paridaens R, Smith I, et al. Letrozole therapy alone or in sequence with tamoxifen in women with breast cancer. N Engl J Med. 2009;361(8):766-76. [ Links ]
29 Cuzick J, Sestak I, Baum M, Buzdar A, Howell A, Dowsett M, et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol. 2010;11(12):1135-41. [ Links ]
30 Russell RG. Bisphosphonates: the first 40 years. Bone. 2011;49(1):2-19. [ Links ]
31 Zhou J, Ma X, Wang T, Zhai S. Comparative efficacy of bisphosphonates in short-term fracture prevention for primary osteoporosis: a systematic review with network meta-analyses. Osteoporos Int. 2016;27(11):3289-300. [ Links ]
32 Colón-Emeric CS, Mesenbrink P, Lyles KW, Pieper CF, Boonen S, Delmas P, et al. Potential mediators of the mortality reduction with zoledronic acid after hip fracture. J Bone Miner Res. 2010;25(1):91-7. [ Links ]
Received: April 22, 2020; Accepted: September 27, 2020











 texto em
texto em 


