My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista de Osteoporosis y Metabolismo Mineral
On-line version ISSN 2173-2345Print version ISSN 1889-836X
Rev Osteoporos Metab Miner vol.12 n.4 Madrid Oct./Dec. 2020 Epub Apr 05, 2021
https://dx.doi.org/10.4321/s1889-836x2020000400005
ORIGINALS
Cx43 and primary cilium involvement in osteocyte activity
3Applied Medicine Institute of San Pablo-CEU University (Spain)
4Department of Basic Medical Sciences. Faculty of Medicine. San Pablo-CEU University. Madrid (Spain)
Objetivo
Bone tissue can adapt to environmental stimuli by altering its morphology and metabolism. Different bone cells communicate with each other through communicating junctions (CJs). Connexin 43 (Cx43) is the most abundant CJ protein with key functions in signal transduction and in response to hormonal and mechanical stimuli. Another mechanosensor element of osteocytes is the primary cilium, formed by microtubules and which develops in the cell cycle’s G0 phase.
Our study aims to determine Cx43 and primary cilium involvement in osteocytic activity, to analyze the possible interaction between these two mechanosensors and to assess the role they play in the detection and response of osteocytes to mechanical stimuli and stimulation of the parathormone type 1 receptor (PTH1R) by its ligand, the parathormone-related protein (PTHrP) (1-36).
Material and methods
The control MLO-Y4 (Cx43 +/+) osteocyte cell line was compared to Cx43-deficient MLO-Y4 (Cx43 -/-). The expression analysis of intraflagellar transport protein 88 (IFT88), Cx43 and phosphorylation of the extracellular signal regulatory kinase (P-ERK) was determined by Western blot. To characterize the possible colocalization between the primary cilium and Cx43, an immunofluorescence was carried out. To simulate mechanical stimulation in vitro, cells were subjected to mechanical stress of 10 dynes/cm2 by fluid flow for 10 minutes.
Results
The results obtained show that the number of cells with primary cilium does not vary due to the expression of Cx43 (p = 0.089). In cells with Cx43 presence, mechanical stimulation by fluid flow and PTHrP increase the phosphorylation of extracellular signal-regulated kinases (ERK) compared to unstimulated cells (p = 0.049 and p = 0.011, respectively).
Conclusions
The primary cilium and Cx43 act as mechanosensing elements for osteocytes. Deficiency in Cx43 does not influence ciliogenesis or activation by mechanical stimulation of pro-survival signaling pathways in osteocytes.
Key words osteocytes; connexin 43; primary cilium; mechanical stimulus; PTHrP
INTRODUCTION
Bone tissue has the ability to adapt to surrounding environmental stimuli by altering its morphology and metabolism1.
The development, remodelling and repair of this tissue are dynamic processes, regulated by the joint activity of bone cells (osteocytes, osteoblasts and osteoclasts). Osteocytes are the most abundant type of cells in the bone. They are located in the mineralized bone matrix, forming a large cellular intercommunication network, called osteocyte lacuno-canalicular system (OLCS). Osteocytes are the main mechanosensory cells in the bone2. They can detect mechanical stimuli in the environment and communicate this signal to effector cells (osteoblasts and osteoclasts) and have different mechanosensory structures: ion channels, integrins3, parathyroid hormone receptor type 1 (PTH1R) ligands, connexins4 and primary cilia. Some of these mechanosensors have been found to interact with each other, allowing the integration of multiple extracellular signals3.
Mechanical stimuli regulate bone remodelling. The deregulation of this process produces osteoporosis, a condition characterized by decreased bone mass and increased bone frailty5.
Osteocytes respond to mechanical stimulation by activating different signalling pathways, such as Winglesstype protein (Wnt)HPPβ-catenin, and mitogen-activated protein kinases (MAPK) and Hedghog (HH). In this work, we analized some of the molecules acting in these signalling pathways, specifically P-ERK and ERK proteins. Interaction among the cells constituting the bone tissue is essential for bone tissue homeostasis6.
Cell communication via gap junctions (GJs) is one of the most important of these interactions, as it happens between cytoplasms of adjacent cells and, therefore, allows intercellular diffusion of small molecules7. GJs not only serve as passive channels, they also intervene in the regulation of different signaling routes8.
Cxs are transmembrane proteins, named after their molecular weight, from 26 to 59 kDa. Cx43 is the most abundant protein in the GJs of bone cells. Connexins, in particular Cx43, interact with structural and signalling molecules, regulating cellular functions9,10.
Primary cilia are microtubule-based structures in which numerous receptor channels and proteins are located, which allow the cilia to act as a mechanosensors11,12. PTH1R is a G-protein-coupled receptor (GPCR), which is expressed in primary cilia and plays a fundamental role in mechanical signal transduction in MLO-Y413 cells. This receptor has two widely characterized ligands: PTH and PTHrP (parathormone-related protein). Both PTH and PTHrP have effects on bone formation and are used as anabolic agents in the treatment of osteoporosis13,14.
It has also been shown that stimulation by PTHrP and mechanical stimulation by fluid flow induce the activation of ERK, thus preventing the increase of osteocyte apoptosis15.
In the present study, it was hypothesized that primary cilia and Cx43 act jointly in the regulation of signalling pathways involved in cell survival and in cell adhesion capacity. The expression of primary cilia was determined both in Cx43 +HPP+ and Cx43 -HPP- cells and the non-colocalization of these two mechanosensors. Therefore, it is suggested that Cx43 deficiency is not involved in the development of the primary cilia, but could influence other aspects, such as their functionality, length or intra-flagellar transport.
Likewise, the cellular response of osteocytes (phosphorylation of ERK) was analyzed after stimulating PTH1R, both with PTHrP, obtaining a greater increase in P-ERK in Cx43 -HPP- cells as opposed to in Cx43 +HPP+, and mechanically, which produced an increased P-ERK expression regardless of Cx43 deficiency.
MATERIALS AND METHODS
1. Cell cultures
In this project, we worked with the continuous line of murine long bone MLO-Y4 osteocytes Cx43 +HPP+, as a control, and deficient in Cx43 (Cx43 -HPP-), which were ki1. Cell culturesndly provided by Dr. L. I Plotkin.
The cells were cultured at a concentration of 24,000 cellsHPPcm2, with ɑ-Modified Eagle's Medium (ɑ-MEM) (GibcoTM, Thermo Fisher Scientfic, Spain), supplemented with 2.5% calf serum (Calf Serum; CS), 2.5% fetal bovine serum (FBS), 1% L-Glutamine, 1% PenicillinHPPStreptomycin and Puromycin (from Streptomyces alboniger, Sigma Aldrich, BioReagent, Merck, Spain) at a concentration of 10 μgHPPml.
To promote the development of the primary cilium, the cells were grown in depletion medium, composed of ɑ-MEM (GibcoTM) supplemented with 1% penicillinHPPstreptomycin and puromycin at a concentration of 10 μgHPPml, for 24 hours.
All surfaces on which these cells were cultured had to be previously collagenized, using for this matter type I collagen at 0.01% acetic acid, to simulate the collagen matrix where the osteocytes are embedded in vivo. Cells were kept at 37ºC and 5% CO2.
2. Western blot
The cellular total protein was extracted using RIPA buffer (Sigma-Aldrich, Merck, Spain), supplemented with protease and phosphatase inhibitors (Calbiochem, Merck, Spain).
Subsequently, these proteins were quantified using bicinchoninic acid (BCA) (PierceTM BCA Protein Assay Kit, Thermo Fisher Scientfic, Spain), which generates a colorimetric reaction detectable at 562 nanometres (nm). To carry out the reading, the Varioskan Flash plate reader (Thermo Fisher Scientfic) was used, with SkanIt Software 2.4.3 RE.
Protein extracts (20 µg) were separated by means of a 10% polyacrylamide gel under reduced conditions. They were later transferred to a nitrocellulose membrane (Bio-Rad, Hercules, California, USA). The blocking was carried out with 5% milk powder, in Tris-saline buffer with 0.05% Tween20 (TTBS), for 1 hour under stirring at room temperature. The following primary antibodies were then stirred between 15-18 hours at 4ºC: anti-Phosphop-44HPP42 MAPK (Erk1HPP2) (Cell Signaling, Beverly, Massachusetts, USA), anti-p44HPP42 MAPK (Erk1HPP2), anti-Cx43 (Sigma Aldrich, ST. Louis, Missouri, USA) and anti-tubulin (Sigma Aldrich). These are all polyclonal antibodies produced in rabbits, except for anti-tubulin which is a monoclonal antibody produced in mice. It was then incubated for one hour at room temperature, with the corresponding IgG coupled to peroxidase, and the membrane was developed by chemiluminescence with ClarityTM Western ECL substrate (Bio-Rad, Life Science Research, Spain). The intensity of the bands was quantified by densitometry, using the DNR Bio Imaging System MF ChemiBIS3.2 and Gelcapture and QuantityOneTM (Bio-Rad) programs.
3. Immunofluorescence
30,000 cellsHPPwell were cultured from the multiwell plates (Falcon®, Thermo Fisher Scientfic, Spain). The cells were grown until they reached 80% confluence; and subsequently depletion medium was added for 24 hours to induce the formation of the primary cilia. The cells were then fixed with 4% paraformaldehyde (PFA) and permeabilized with Triton X-100 at 0.5%. Next, the blocking solution, composed of bovine serum albumin (BSA) at 10%, supplemented with goat serum at 5%, was added for 1 hour. After that, the following primary antibodies were stirred for 15-18 hours at 4ºC: polyclonal anti-Cx43 produced in rabbits (Sigma Aldrich) and monoclonal anti-ɑ acetylated tubulin produced in mice (Sigma Aldrich), to observe the primary cilium. The secondary antibodies were then arranged: Alexa fluor® 488 goat anti-mouse cilium (Invitrogen Molecular probes, Thermo Fisher ScientificTM, Spain), and Cx43, Alexa fluor® 568 anti-rabbit IgG (Life technologies, Thermo ScientificTM, Spain) (1: 1000 dilution in BSA at 10% and goat serum at 5%). After 1 h of incubation, 4'-6-diamidino2-phenylindole (DAPI) was added. Nuclei, the primary cilia and Cx43 were seen on the fluorescence microscope (Leica CTR 6000). The merging of the individual images of the primary cilium, Cx43 and cell nuclei into one single image was performed using the ImageJ program.
4. Mechanical stimulation by fluid flow (FF) and by PTHrP
To perform stimulation by FF and PTHrP, cells were cultured on teflon-bound glass slides at a density of 25,000 cellsHPPcm2. The FF technique is based on the constant bombing of culture medium over the cell monolayer using a peristaltic pump (Flexcell International Corp., Hillsborough, North Carolina, USA.) at 10 dynesHPPcm2 in a hermetically closed circuit for 10 minutes. The time and frequency settings were established using the Master Flex Peristaltic Pump 2010 program. On the other hand, stimulation was performed with PTHrP (1-36) (Bachem, Bubendorf, Switzerland) at a concentration of 10-7 molar (M), for 10 min. The same number of cultures, but with cells that were not subjected to any stimuli, served as static controls (SC).
5. Statistical analysis
In the statistical analysis of the results, data are expressed as mean ± standard deviation of at least two experiments carried out in triplicate. It was performed using the GraphPad Prism 8 program (GraphPad software, La Jolla, California, USA). To compare means between two groups, the nonparametric Mann-Whitney test was carried out, and to compare means of more than two groups, the non-parametric Kruskal-Wallis test was used. For mulptiple pairwise comparison we used Dunn’s test. The established confidence interval was 95% in all statistical tests. So the results with a value of p<0.05 were considered statistically significant.
RESULTS
1. Effect of Cx43 on the development of the primary cilium in Cx43 +HPP+ and Cx43 -HPP- cells
The results obtained by Western blot indicate that in Cx43-deficient cells the expression of this protein decreases significantly in comparison with control cells Cx43 +HPP+ (W=0, p=0.029), which allows us to confirm the deficiency of this protein (Figure 1). In addition, the presence of the primary cilium was characterized by the expression analysis of the IFT88 protein. IFT88 was used as a marker for the primary cilium presence, since it is a highly abundant protein in this organelle, as it is involved in intraflagellar transport, needed for ciliogenesis18. We observed that the IFT88 protein expresses in a similar way, regardless of the deficiency in Cx43 (W=4, p=0.343).
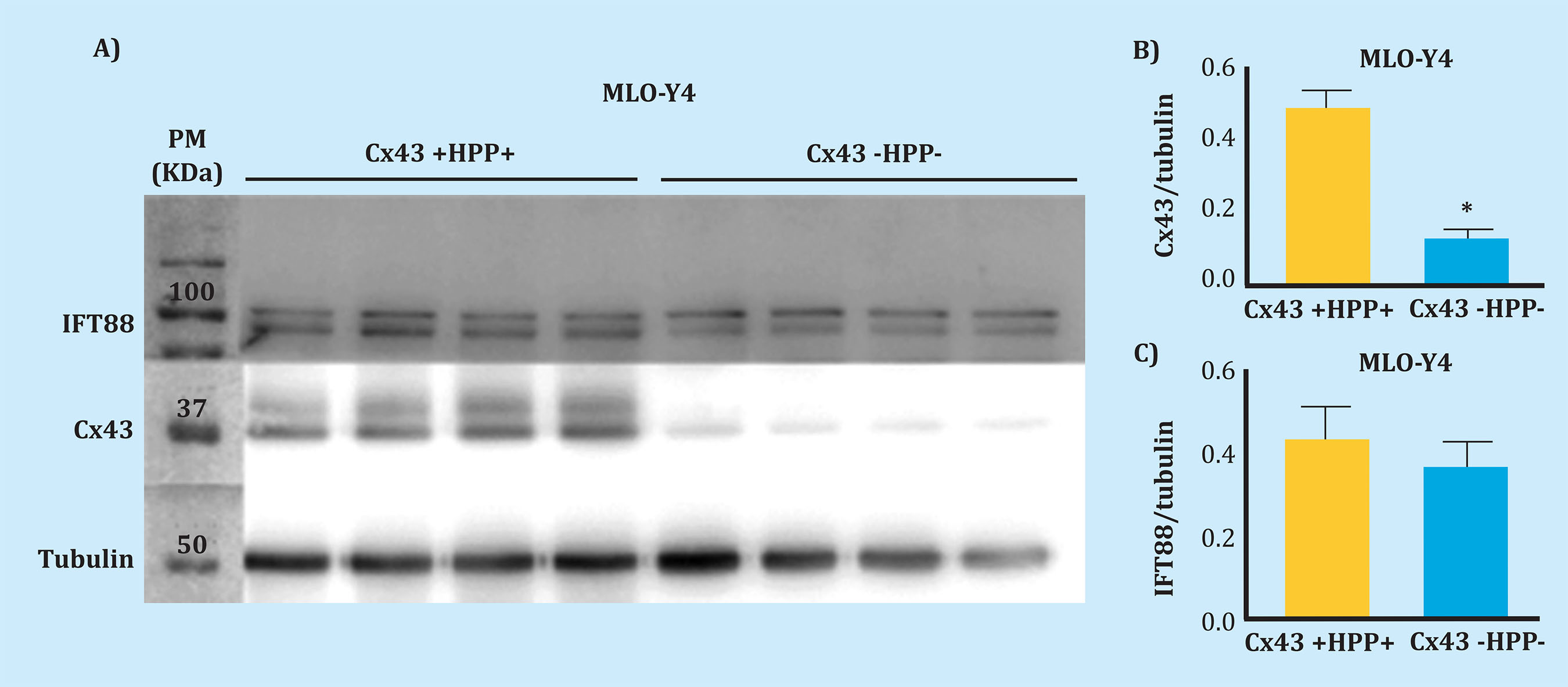
Figure 1. Expression of Cx43 and IFT88. A) Result of the nitrocellulose membrane development for the analysis of Cx43, IFT88 and tubulin as loading control; total cellular protein extracts were used (25 µg). The molecular weight (MW) marker was placed in the first lane; the next four lanes belong to four replicas of control MLO-Y4 cells (Cx43 +HPP+) and the last four correspond to four replicas of Cx43-deficient MLO-Y4 cells (Cx43 -HPP-). B) Mean ± standard deviation of Cx43HPPtubulin. C) Mean ± standard deviation of IFT88HPPtubulin. Protein levels normalized in contrast to tubulin *p<0.05
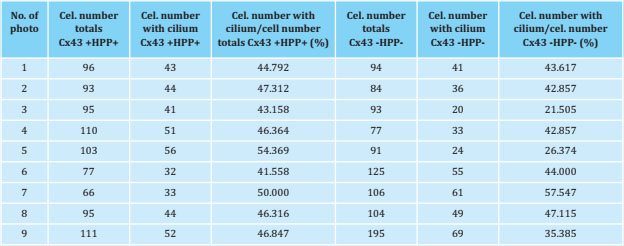
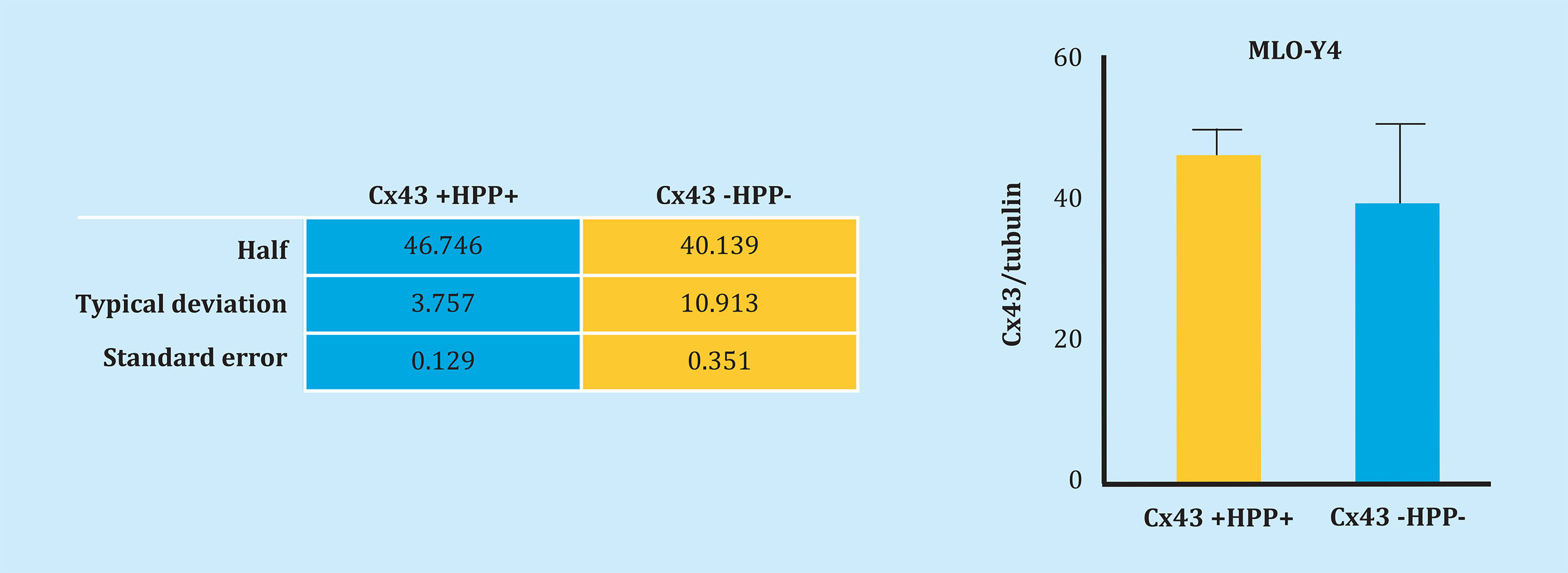
Likewise, Cx43 and primary cilia were analyzed by immunofluorescence to try to determine the possible interaction between these two mechanosensors. Figure 2 shows both Cx43 +HPP+ and Cx43 -HPP- cells develop a primary cilium, which is evidenced by the presence of the primary anti-α-acetylated tubulin antibody, which originates from the cell surface. Also, it can be seen that Cx43 appears mainly in the cell membrane, possibly forming GJs. Furthermore, it appears that the primary cilium does not co-localize with Cx43. On the other hand, we performed counts of the cells with primary cilium from nine photographs taken in different fields using the fluorescence microscope. In each image the number (No.) of total cells (cel.) and the number of cells with a presence of primary cilium were quantified, and we calculated the ratio (No. of cells with ciliumHPPNo. of total cells), both for the Cx43 +HPP+ and for Cx43 -HPP- cell lines (Table 1 and Figure 3). In these results, we found that the number of cilia formed did not significantly differ (p=0.089) between the two cell lines (Cx43 +HPP+ and Cx43 -HPP-).
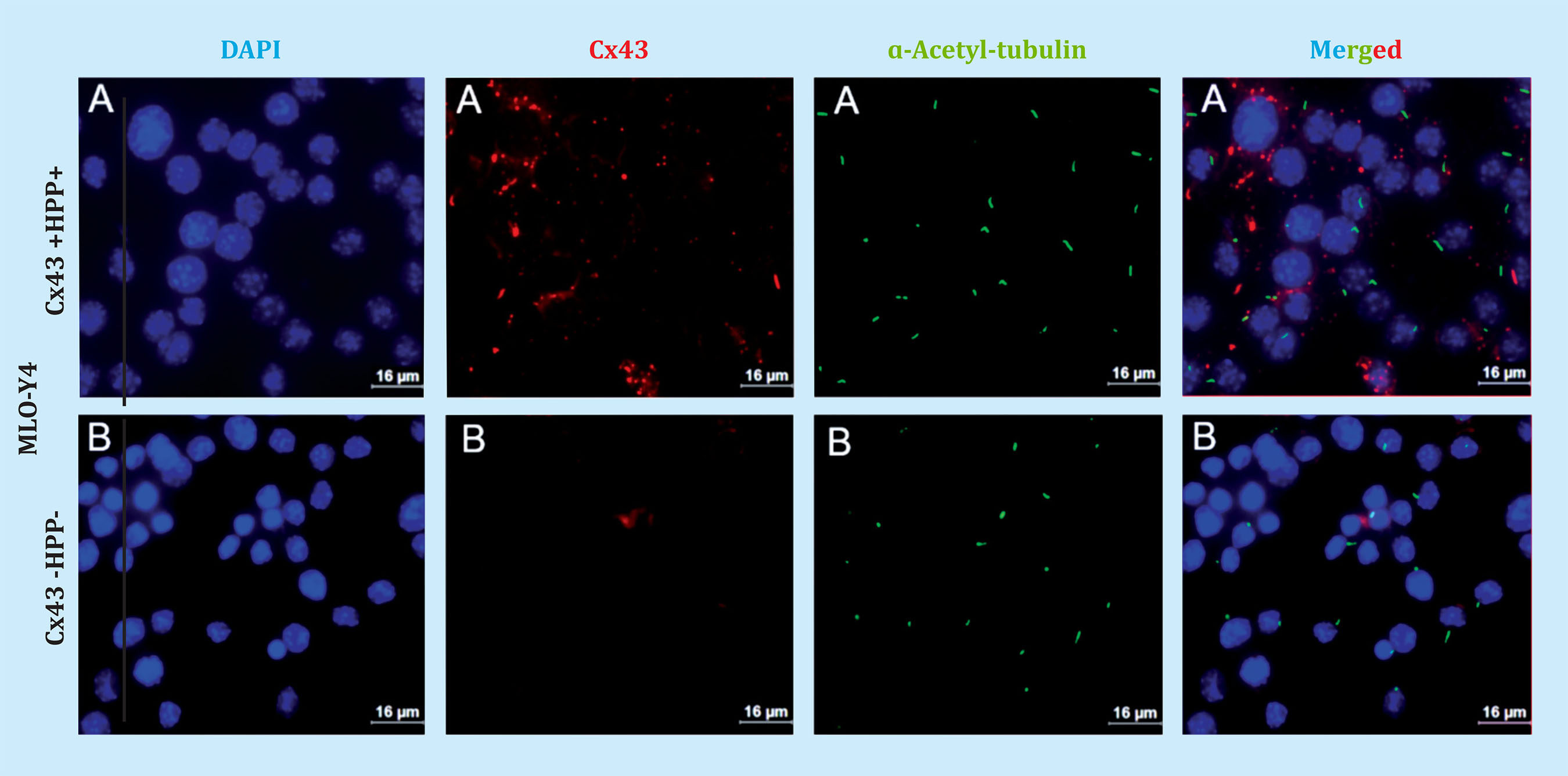
Figure 2. Immunofluorescence performed with osteocytes of the MLO-Y4. A) Cx43 +HPP+ and B) Cx43 -HPP- line. Cell nuclei were stained with DAPI (blue), anti ɑ-acetyl-tubulin antibodies were used to mark the primary cilium (green), anti-Cx43 antibody to mark Cx43 (red) and merged. The images were taken with an immersion objective at 63X. Scale bar = 16 μm
2. Effect of mechanical stimulation and PTHrP on Cx43 +HPP+ and Cx43 -HPP- cells
In several studies it has been determined that mechanical stimulation inhibits osteocyte apoptosis, through a mechanism that involves phosphorylation of MAPKs as ERKs16. The aim of this experiment was to analyze whether Cx43 deficiency altered the effect of mechanical stimulation on osteocytes, and to determine whether Cx43 is involved in the activation of the PTH1R receptor, after stimulated by one of its PTHrP ligands. The effects triggering mechanical stimulation and PTHrP stimulation in MLO-Y4 cells, the P-ERK expression was analyzed using Western blot.
The Kruskal-Wallis test was applied to establish the differences between the Cx43 +HPP+ and Cx43 -HPP- groups for the conditions: SC, FF and PTHrP. The test result pointed at differences between some of the groups (p=0.0005). To determine between which groups, Dunn’s Multiple Comparison Test was performed, showing that both mechanical stimulation (FF) (p=0.049) and PTHrP stimulation (p=0.017) induce a significant increase in ERK phosphorylation in contrast to non-stimulated cells (static control, SC); in the MLO-Y4 Cx43 +HPP+ cell line. This result shows that extracellular stimuli favour the activation of the SrcHPPERK signaling pathway, which promotes cell survival. In the case of MLO-Y4 Cx43 -HPP- we observed that P-ERK does not increase significantly in contrast to CE (p=0.955) after mechanical stimulation (FF). However, when stimulated with PTHrP, ERK phosphorylation increases significantly (p=0.025) (Figure 4).
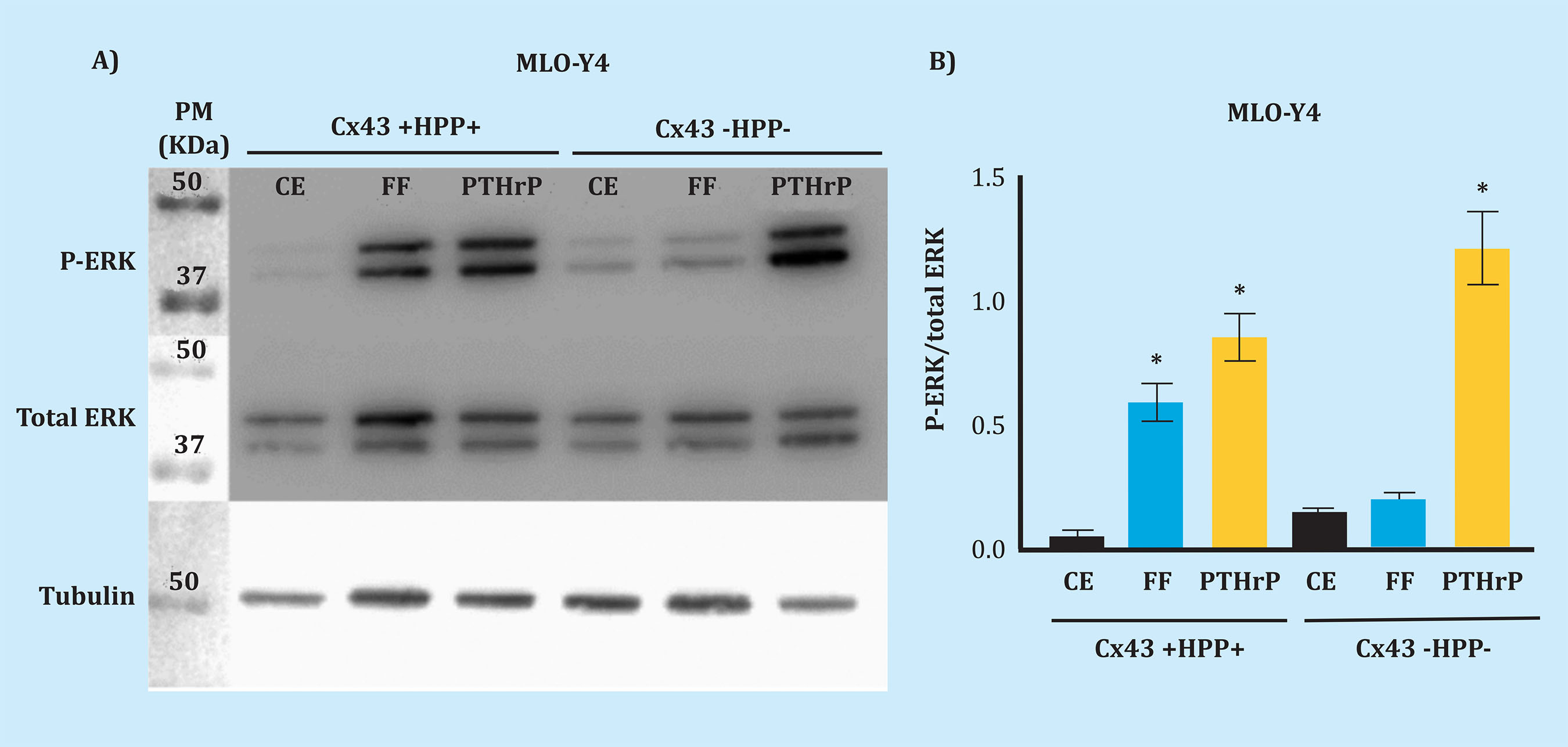
Figure 4. P-ERK analysis after mechanical stimulation and with PTHrP. After mechanical stimulation and stimulation with PTHrP, changes in protein levels were evaluated by Western blot, and those regarding P-ERK in total cell protein extracts, cell lines Cx43 +HPP+ and Cx43 -HPP-, stimulated or not (static control, SC). Total ERK and tubulin were used in order to normalize. Results are expressed as mean ± standard deviation of a duplicate experiment of each condition vs. SC
DISCUSSION
Connexins and primary cilia are bone cells’ mechanosensory elements which play a fundamental role in the stimuli detection and signal transmission3.
The results obtained by Western blot confirm that the Cx43 -HPP- cell line used in the experiment was deficient in this protein, since the level of Cx43 expression decreased significantly compared to that on the Cx43 +HPP+ line. The IFT88 protein expression was also analyzed, since it was used as a marker for the presence of the primary cilia in previous studies17. According to the results obtained, it can be concluded that there are no significant differences in the IFT88 protein expression between the Cx43 +HPP+ and Cx43 -HPP- lines. The results of the IFT88 analysis by Western blot alone do not allow us to ensure that primary cilia develops correctly, because the IFT88 protein could be expressing itself in another cell compartment different from the primary cilia. Therefore, to verify whether the deficiency in Cx43 influences the formation of the primary cilia, the expression of this organelle and of Cx43 was analyzed by immunofluorescence. Both Cx43 +HPP+ cells and Cx43 -HPP- cells were observed to develop primary cilia; Likewise, it was found that the number of cilia formed did not differ meaningfully between the two cell lines. Furthermore, it seems to be observed that the Cx43 and the primary cilium do not co-localize; and in Cx43 +HPP+ cells, Cx43 is found in the plasma membrane of the cell, which would be the expected location, since it is where it forms GJs.
Previous research has shown that MLO-Y4 cells are an optimal model for mechanical stimulation studies16. However, in order to extrapolate the results obtained in these in vitro studies to the authentic in vivo conditions, it is necessary to work with mechanical stimuli that re-produce and generate responses similar to those that occur in the physiological situation. Currently, fluid flow (FF) over a monolayer of osteocytic cells is the technique that most closely approaches this situation18.
The molecular target chosen as an indicator of the viability of the MLO-Y4 line was P-ERK, because its activation after mechanical stimulation is an indicator of survival in osteocytic cells16,18. In accordance with these investigations, mechanical stimulation by FF was found to induce an increase in the expression of P-ERK16,18. Thus, FF promotes the survival of MLO-Y4 cells.
On the other hand, the PTHrPHPPPTH1R system is also essential to regulate bone remodelling. Preliminary results suggest that PTH1R is key in the anabolic bone response consequent to mechanical stimulation in vivo. This receptor acts as a mechanoreceptor in osteoblast cells13. The results of the study carried out indicate that the exogenous administration of PTHrP (1-36) (PTH1R ligand) protects against apoptosis in a similar way to that of mechanical stimulation in osteocytes, since it induces an increase in the expression of P- ERK. Similarly, previous studies found that PTHrP, like PTH, has anti-apoptotic properties in osteocytes16.
CONCLUSIONS
The number of cells with primary cilium does not vary due to the expression of Cx43.
The primary cilium and the Cx43 act as osteocytes mechanosensors.
The fluid flow-induced mechanical stimulation promotes the survival of MLO-Y4 cells, regardless of the deficiency in Cx43, since it causes an increase in the P-ERK expression, both in Cx43 +HPP+ and Cx43 -HPP- cells.
Exogenous administration of PTHrP (1-36) (PTH1R ligand) produces an increase in P-ERK in Cx43 +HPP+ and Cx43 -HPP- cells. However, this increase is much higher in Cx43 -HPP- cells. Therefore, we suggest Cx43 is inhibiting the PTH1R receptor, which, after binding its PTHrP ligand, causes the activation pathway in which P-ERK is involved not to fully trigger.
Bibliografía
1 Matthew RA, Kelly K. Skeletal Imaging. In: Burr DB, Allen MR. Basic and Applied Bone Biology. 1ª ed. USA: Academic Press; 2013. p. 105-209. [ Links ]
2 Rupp M, Merboth F, Daghma DE, Biehl C, El Khassawna T, Heiß C. Osteocytes. Z Orthop Unfall. 2019;157(2):154-63. [ Links ]
3 Geoghegan IP, Hoey DA, McNamara LM. Integrins in osteocyte biology and mechanotransduction. Curr Osteoporos Rep. 2019;17(4):195-206. [ Links ]
4 Jiang JX, Cherian PP. Hemichannels formed by connexin 43 play an important role in the release of prostaglandin E(2) by osteocytes in response to mechanical strain. Cell Commun Adhes. 2003;10(4-6):259-64. [ Links ]
5 Tu KN, Lie JD, Wan CKV, Cameron M, Austel AG, Nguyen JK, et al. Osteoporosis: A Review of Treatment Options. P T. 2018;43(2): 92-104. [ Links ]
6 Plotkin LI, Laird DW, Amedee J. Role of connexins and pannexins during ontogeny, regeneration, and pathologies of bone. BMC Cell Biol. 2016;17(1):19-28. [ Links ]
7 Loiselle AE, Jiang JX, Donahue, HJ. Gap junction and hemichannel functions in osteocytes. Bone. 2013;54(2):205-12. [ Links ]
8 Xu H, Gu S, Riquelme MA, Burra S, Callaway D, Cheng H, et al. Connexin 43 channels are essential for normal bone structure and osteocyte viability. J Bone Miner Res. 2015;30(3):436-48. [ Links ]
9 Solan JL, Lampe PD. Spatio-temporal regulation of connexin43 phosphorylation and gap junction dynamics. Biochim Biophys Acta Biomembr. 2018; 1860(1):83-90. [ Links ]
10 Giepmans BN. Role of connexin43-interacting proteins at gap junctions. Adv Cardiol. 2006;42(1):41–56. [ Links ]
11 Dbouk HA, Mroue RM, El-Sabban ME, Talhouk, RS. Connexins: a myriad of functions extending beyond assembly of gap junction channels. Cell Commun Signal. 2009;7(4):2-15. [ Links ]
12 Hoey DA, Downs ME, Jacobs CR. The mechanics of the primary cilium: an intricate structure with complex function. J Biomech. 2012;45(1):17-26. [ Links ]
13 Schwartz EEA, Leonard ML, Bizios R, Bowser SS. Analysis and modeling of the primary cilium bending response to fluid shear. Am J Physiol. 1997;272 (1):132-8. [ Links ]
14 Maycas M, Ardura JA, Castro, LF, Bravo B, Gortázar A R, Esbrit P. Role of the parathyroid hormone type 1 receptor (pth1r) as a mechanosensor in osteocyte survival. J Bone Miner Res. 2015; 30(7):1231-44. [ Links ]
15 Sleeman A, Clements JN. Abaloparatide: A new pharmacological option for osteoporosis. Am J Health Syst Pharm. 2019;76(3):130-5. [ Links ]
16 Maycas M. (2016). Mecanismos moleculares implicados en la mecanotransducción osteocítica. Alteraciones en la osteopatía diabética y efecto compensador de la proteína relacionada con la parathormona (PTHrP). Tesis Doctoral. Universidad Autónoma de Madrid. Páginas 4-138. [ Links ]
17 Plotkin LI, Mathov I, Aguirre JI, Parfitt AM, Manolagas SC, Bellido T. Mechanical stimulation prevents osteocyte apoptosis: requirement of integrins, Src kinases, and ERKs. Am J Physiol Cell Physiol. 2005;289(3):633-43. [ Links ]
18 Yuan X, Serra RA, Yang S. Function and regulation of primary cilia and intraflagellar transport proteins in the skeleton. Ann N Y Acad Sci. 2014;1335 (1):78-99 [ Links ]
Received: November 09, 2020; Accepted: January 30, 2021











 text in
text in