Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista de Osteoporosis y Metabolismo Mineral
versão On-line ISSN 2173-2345versão impressa ISSN 1889-836X
Rev Osteoporos Metab Miner vol.13 no.2 Madrid Jun. 2021 Epub 16-Ago-2021
https://dx.doi.org/10.4321/s1889-836x2021000200003
ORIGINALS
Influence of breastfeeding on bone mineral metabolism after menopause
1Research Group on Osteoporosis and Mineral Metabolism, University of Las Palmas de Gran Canaria. Las Palmas de Gran Canaria (Spain)
2Department of Mathematics, University of Las Palmas de Gran Canaria. Las Palmas de Gran Canaria (Spain)
3Department of Medicine, University of Seville. Seville (Spain)
4Gynecology and Obstetrics Service, Maternal and Child Hospital. Las Palmas de Gran Canaria (Spain)
5Bone Metabolic Unit, Insular University Hospital. Las Palmas de Gran Canaria (Spain)
Objetive
Lifestyle and gynecological history appear to influence bone mineral metabolism. There are conflicting data on the possible effects of breastfeeding on the subsequent development of densitometric osteoporosis or the development of fragility fractures. The objective of this study was to assess these effects.
Material and methods
Observational, cross-sectional, open study, carried out in 758 postmenopausal women who were classified into two groups, depending on whether they had breastfed their children or not. Data were collected on lifestyles, gynecological history and fragility fractures. They underwent a general analysis, with renal and hepatic function, lipids, ions, as well as biochemical markers of bone remodeling, parathyroid hormone (PTH) and vitamin D (25HCC). Bone mineral density (BMD) was determined in the lumbar spine and in the proximal extremity of the femur by dual Xray absorptiometry (DXA). Likewise, a quantitative ultrasound (QUS) measurement was performed on the calcaneus of the dominant foot. The raw data, after being compared by groups, were adjusted by applying the propensity score matching method, making a more precise comparison of the variables studied.
Results
The results prior to the application of the propensity score were adjusted for age and body mass index (BMI), since in the baseline study there were significant differences in these variables between both groups (prevalence of hip fractures and kyphosis and in the following biochemical parameters: specifically uric acid, glucose, HDL-cholesterol, triglycerides and phosphorus). These differences disappeared after adjusting for the variables that were included in the model by the applied linear logistic regression. After adjusting with the propensity score matching and with the finally obtained linear regression model, no influence of breastfeeding was obtained on bone mineral density, on the prevalence of densitometric osteoporosis or on the appearance of fragility fractures after menopause.
Conclusions
Breastfeeding is not associated with higher or lower bone mineral density values, the prevalence of densitometric osteoporosis, or the presence of fragility fractures.
Key words breastfeeding; pregnancy; osteoporosis; fragility fractures; propensity score matching; bone density
INTRODUCTION
Osteoporosis is defined as a skeletal disease in which there is a decrease in bone strength that leads to an increased risk of fracture, usually due to mild trauma1. Although any fracture can be observed in clinical practice, with the exception of the skull bones, the most prevalent is the vertebral one and the most serious that of the proximal extremity of the femur2, given its significant morbidity and mortality3. Genetic, anthropometric, nutritional and lifestyle factors4-11 influence the appearance of fragility fractures or osteoporotic fractures, but also gynecological and obstetric factors12. Among them, breastfeeding reportedly exerts an essential reproductive function in women and protects the mother from developing many diseases, such as cancer or diabetes11-14.
Its effect on bone mineral metabolism is less defined, however, and published results are often contradictory. Some of these studies indicate that prolonged breastfeeding could be associated with an increase in bone mineral density (BMD) and a lower prevalence of osteoporosis in postmenopausal women12-16, while others suggest precisely the opposite, that prolonged breastfeeding is a risk factor for the appearance of osteoporosis and fragility fractures17-21. Finally, reports have also been published that do not find any effect, neither beneficial nor harmful22-24.
Therefore, we have carried out the present study in a population of postmenopausal women to establish whether or not breastfeeding is associated with the subsequent appearance of densitometric osteoporosis and the presence of fragility fractures, with the particularity that the propensity score matching method was used. This provides a more precise comparison of the variables studied in the established groups, making them more homogeneous as we will describe in more detail in this paper.
MATERIAL AND METHODS
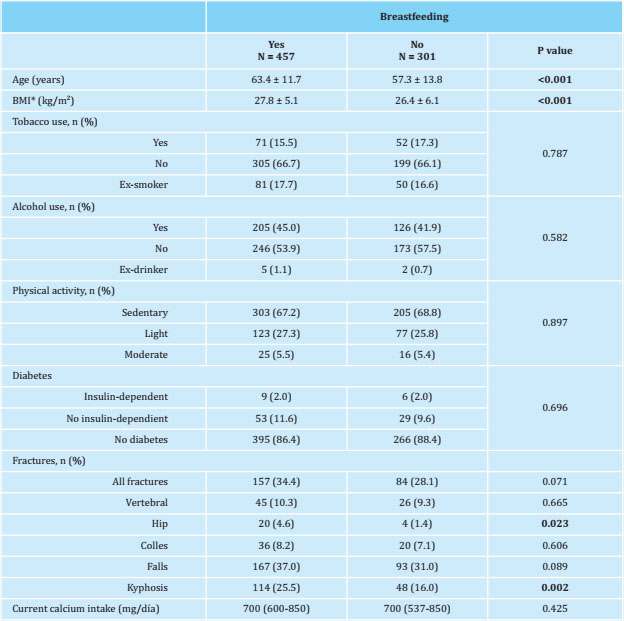
A total of 758 women were included, who were studied in the Bone Metabolic Unit of the Insular University Hospital in the period between 2016-2020. They were informed of the objectives of the study and gave their informed consent. All completed a questionnaire, previously validated and used in other similar clinical studies on osteoporosis25,26. They also underwent a basic physical examination that included height and weight measurements to then calculate their body mass index (BMI). Subsequently, they were grouped into women who had breastfed (cases) and women who did not (controls).
Sample collection and laboratory techniques
Blood and urine samples were collected in the morning, between 8:00 and 9:00 am, after an overnight fast. Blood was collected in the appropriate specific tubes for each determination, with the least possible venous compression, and was centrifuged at 1,500 g for 10 minutes; the serum was separated into aliquots and stored within one hour from the extraction at -20ºC until the biochemical analyzes were carried out, although most of them were carried out the same day as the extraction. Glucose, urea, creatinine, calcium, inorganic phosphorus, total proteins, total cholesterol and its fractions and triglycerides were measured using standardized and automated colorimetric techniques on an autoanalyzer (Kodak Ektachem Clinical Chemistry Slides).
Serum calcium was corrected according to total proteins by means of the following formula:
Determination of ultrasound values in the calcaneus
Ultrasonographic parameters were estimated in the calcaneus of the dominant foot using a Sahara® Hologic® ultrasonographer (Bedford, Massachusetts, USA). This device measures both the broadband ultrasound attenuation (BUA) and the speed of sound (Speed of sound, SOS) in the region of interest of the calcaneus. The BUA and SOS values are combined into a single parameter called the quantitative ultrasound index (QUI), also known as the consistency or stiffness index, which is obtained by means of the formula:
The T-score values were calculated from the values published as normal for the Spanish population27.
Bone mineral density (BMD)
BMD was measured by dual radiological absorptiometry (DXA), both in the lumbar spine (L2-L4) and in the proximal extremity of the femur, with a Hologic Discovery® densitometer (Hologic Inc, Waltham, USA), whose accuracy is 0.75-0.16%. The measurements were made by the same operator, so there was no inter-observer variation. The T-score values were calculated from the values published as normal for the Canary Island population28.
Diagnosis of osteoporosis and fragility fractures
Osteoporosis was considered to exist when a T-score equal to or less than -2.5 was obtained in any of the 3 anatomical locations where bone mineral density was determined: lumbar spine L2-L4, femoral neck or total hip.
The existence of a fragility fracture was diagnosed when they occurred without a trauma to justify it or when a maximum fall from the height of the woman in question. The fractures were confirmed by medical reports available in their medical history: emergency services, trauma, rehabilitation, or after analyzing x-rays.
Ethics
Our study was carried out in accordance with the standards of the Declaration of Helsinki29 and was approved by the Ethics Committee of the Insular University Hospital. All patients were informed of the objectives of the study and their informed consent was requested.
Statistic analysis
Univariate analysis
Initially, we carried out an analysis of the numerical variables, studying whether or not they followed a normal distribution. Later we carried out a descriptive study. Categorical variables were summarized by percentages, and numerical variables by means and standard deviations if they followed normality, or as median and their interquartile range (percentiles = 25th – 75th) if they did not. To study the possible associations between categorical variables, the Chi-square test (χ2) was used and the odds ratio (OR) was used as a measure of association, which was estimated using a 95% confidence interval (95% CI). In those cases in which there were cells with less than 5 cases, Fischer's exact test was applied.
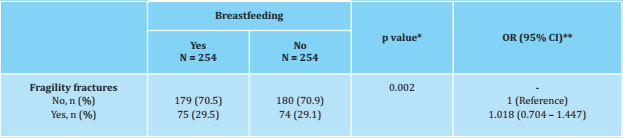
Table 1. Baseline characteristics of the women studied
The data are expressed as means ± standard deviations, medians (IQR) and frequencies in number (%); * BMI: body mass index.
To evaluate the association between a quantitative variable and a categorical variable, the Student's t-test or ANOVA (if there were more than 2 categories) was used for variables with normal distribution, or the non-parametric Mann-Whitney U test for the non-normal ones. In all cases, the significance level was considered at 5% (p<0.05).
Propensity score matching
To establish the association between breastfeeding and the presence of fragility fractures more precisely and to eliminate the influence of other variables, a similar non-lactating control (matching) was selected for each case of lactating women. This process was based on the method called propensity score matching, which in our case is defined by the conditional probability that breastfeeding is conditioned by those variables that could act as confounding factors. The propensity score was obtained for each patient using logistic regression, in which the final variable was breastfeeding. The co-variates included in the model were selected using the complete enumeration algorithm and the Akaike information criteria (Akaike Information Criterion, AIC).
Matching
Subsequently, we performed an adjusted 1 to 1 analysis without replacement, based on the propensity score of each patient. The caliper or calibrator chosen was 0.7. After adjustment for the propensity score, the baseline characteristics were compared by McNemar's test for binary variables or with the t-test or Wilcoxon, as appropriate in each case, for continuous variables and paired data. The 13 variables selected by the program to be included in the matching were: age, BMI, falls, use of statins or thiazides, uric acid, total cholesterol, HDL-cholesterol, triglycerides, the presence of kyphosis and densitometric values in L2-L4, femoral neck and total hip. Furthermore, we established the success of the propensity score adjustment by balancing the adjustment of the covariates in the two groups using the standardized differences. Those differences less than 10% supported the assumption of equilibrium between the two groups. The level of statistical significance was established at 5% (p<0.05). The data were analyzed using the R program, version 3.6.1 (R Development Core Team, 2019).
Table 2. Bone mineral density values obtained by densitometry (DXA) and ultrasound (QUS), values adjusted for age and BMI and prevalence of densitometric osteoporosis
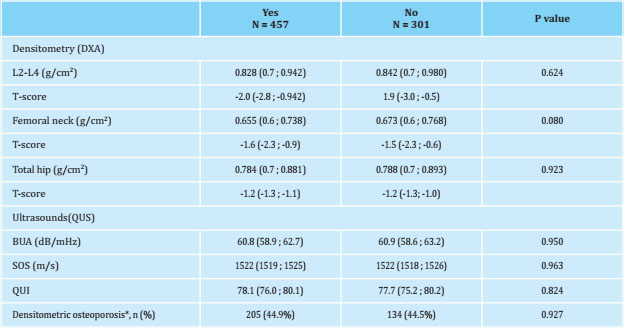
Median (95% CI) adjusted for age and body mass index (BMI); *: presence of a T-score lower than -2.5 in any of the 3 locations where bone mineral density (DXA) was determined, expressed in number (%).
RESULTS
Table 1 of the women included in the study, grouped into women who had breastfed and women who had not. Those who had breastfed were older (63.4 ± 11.7 years versus 57.3 ± 13.8 years, p<0.001) and had a higher BMI, (27.8 ± 5.1 kg/m2 versus 26.4 ± 6.1 kg/m2), were performed after adjusting for these two variables. The prevalence of hip fracture was higher among women who had breastfed significantly, a significance that subsequently disappeared when adjusting for age and BMI.
Table 2 shows the BMD values obtained in the lumbar spine (L2-L4) and in the proximal extremity of the femur with their corresponding T-scores. The ultrasound index values obtained in the calcaneus are also shown, specifically the ultrasound attenuation coefficient (BUA), the speed of sound (SOS) and the consistency index or stiffness (QUI). No statistically significant differences were observed in any of the values obtained between both groups studied. The prevalence of osteoporosis was similar between both groups: 44.9% in women who had breastfed and 44.5% in those who had not, (p=0.927).
Table 3 shows the biochemical values obtained in both groups studied before making the adjustment. Statistically significant differences (p<0.05) are observed in the serum values of uric acid, HDL-cholesterol, triglycerides and phosphorus. All of these differences subsequently disappeared when propensity score matching was carried out.
Table 3. Biochemical data of the patients included in the study, classified according to whether they had breastfed or not, adjusted for age and BMI
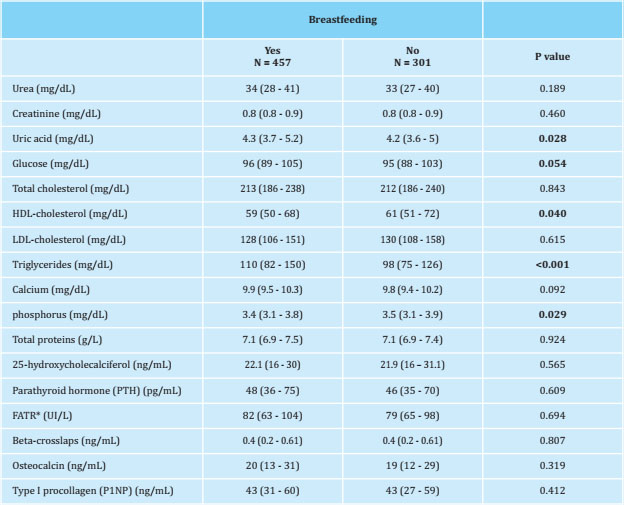
* FATR: tartrate-resistant acid phosphatase.
Table 4 sets out the characteristics of the study patients after matching according to the propensity score of each of them. The variables selected by the program to carry out said matching are shown, which were a total of 13, including all those that had previously shown statistically significant differences in the crude comparisons. As a consequence of this matching, the sample size was substantially reduced to the point that the number of women was finally made up of 254 women in each group. As proof of the correctness of this pairing, it is observed that the standardized differences are less than 10%, which indicates the homogeneity of the variables between both groups.
Table 4. Characteristics of the study women after propensity-score matching
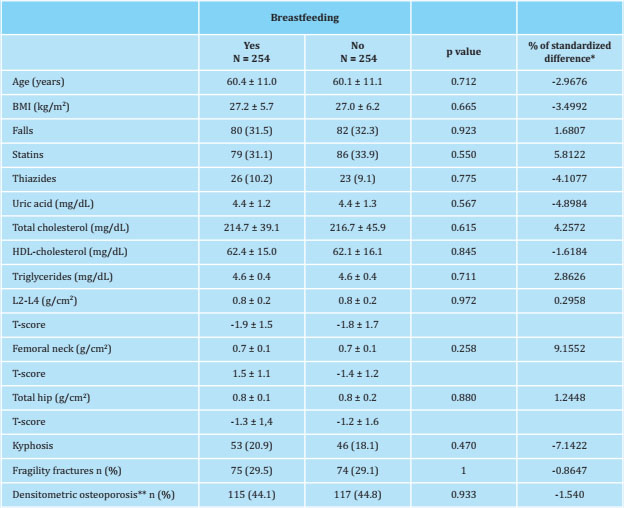
Data are expressed as mean ± standard deviation and frequencies: n (%); the calibrator (caliper) selected was 0.5; *: note that all standardized differences were less than or equal to 10%; **: presence of a T-score lower than -2.5 in any of the 3 locations where bone mineral density (DXA) was determined.
Table 5 shows the data obtained by applying conditional logistic regression for the presence of fragility fractures. After matching, breastfeeding showed no association with fragility fractures.
DISCUSSION
Osteoporosis is a very prevalent disease in which fractures are its only clinical complication2,30. Various risk factors have been implicated in the etiopathogenesis of postmenopausal osteoporosis, related to lifestyle4-7,12, genetics8 and even gynecological history12,14.
One of the etiopathogenic aspects on which there is no consensus is the effect that breastfeeding, which is carried out at a stage of life in which the woman is obviously younger, may have on the subsequent development of osteoporosis after menopause. Some studies suggest that the “negative calcium balance” that would occur during breastfeeding could generate a subsequent loss of bone mass that would manifest itself after menopause with an increased risk of developing densitometric osteoporosis and/or fragility fractures17-21.
In fact, during lactation, the mother supplies the fetus with around 300 mg of calcium daily, the source of which is mainly bone, which produces a loss of between 5-10% of maternal bone mass31, being enough for 3-6 months lactation for this loss to occur32. However, when studying and trying to establish the gynecological and/or obstetric factors that can influence bone mineral metabolism, some authors assess only the presence or absence of pregnancies16, others study the number of pregnancies21 with no shortage of who analyzes the age at which the first pregnancy occurs20. On the other hand, other authors suggest that the organism adapts to this situation, since it is transitory. With several compensatory homeostatic mechanisms, it restores balance in bone mineral metabolism. Other authors suggest that when breastfeeding lasts up to one year, it would be correct to inform the mother of the need for her to acquire nutritional and physical activity habits that facilitate this recovery33,34.
There are also notable differences in the method to be used to assess the effect of breastfeeding on bone mineral metabolism. Some studies analyze changes in BMD16,35, while others consider the risk of developing fragility fractures12,15,36, especially hip fractures18,37. Interestingly, we have not found studies in the literature that analyze the effect of breastfeeding on a very significant aspect of the skeleton, which is bone quality, to such an important extent that some authors consider that it contributes more to fracture risk than the amount measured by BMD38.
Some studies have been carried out in order to know what are the changes in bone mineral metabolism in women at the time they are breastfeeding. Thus, Carneiro et al. suggested the hypothesis that in these women there is an uncoupling between osteoblasts and osteoclasts that leads to a rapid loss of bone mass39.
In a review carried out by Sower on the effect of pregnancy and lactation on bone mineral metabolism, a wide variability is collected in the results obtained in the different publications, which is considered to be largely due to the heterogeneity of the methodology used in these studies40.
A total of 758 women were included in our study, of whom 301 (39.7%) had not breastfed and 457 (60.3%) had. All of them were postmenopausal and in the analysis of their clinical characteristics in the baseline evaluation, we found the existence of statistically significant differences in age and BMI, which is why the densitometric values and the analytical parameters collected in Tables 2 and 3 are compared after adjusting for these two variables.
The distribution of lifestyles, such as tobacco use, physical activity in leisure time and the prevalence of diabetes, showed similar prevalence figures, without obtaining statistically significant differences. In a study by Yan et al. in Chinese women, they found that the differences observed in BMD in postmenopausal women who had breastfed and those who had not, were due to age, BMI and the number of pregnancies and not to the fact of having or not breastfed21. Given the known effect of age and BMI on BMD9 in our study, we decided to adjust for these variables.
Women in both groups, lactating and non-lactating, showed similar BMD values in both the lumbar spine and the proximal end of the femur. Some studies have described that women who breastfeed have lower BMD values than those who do not20,24,32, but there are other authors who find the opposite: a protective effect with higher BMD values and a lower risk of densitometric osteoporosis18,31. A study carried out in Korea in more than one million women41 found that the parameters that were independently associated with an increased risk offracture were the presence of late menarche, early menopause and, therefore, a shorter reproductive period, but not breastfeeding, a finding that concurs with our results.
In the literature consulted, we did not find studies that linked breastfeeding with bone quality assessed by ultrasound in postmenopausal women, and we only found one study carried out in premenopausal women that reported a beneficial effect42.
In our study, no statistically significant differences were observed in the ultrasound indices, so we can accept that breastfeeding has no effect, either positive or negative, on bone quality estimated by these measurements. We consider the statistically significant differences that we have found in the biochemical data to be clinically irrelevant43, as they are within the range of normality established by the laboratory and do not have a clinical impact.
By applying the statistical technique of the propensity score matching method, we achieved a better fit of the women to homogenize both groups. The variables established by the program to be included in the adjustment are shown in table 4 and it can be seen that the standardized difference percentage ranges between -7.1422 and 9.1552. This indicates a very good fit, which has been established by consensus as less than 10%. Although as a consequence of this adjustment, the number of women studied decreased to 254 in each group, thanks to it we were able to establish more precisely, by applying conditional logistic regression, that breastfeeding has no effect on the presence of fragility fractures after menopause.
In conclusion, our study suggests that breastfeeding has no positive or negative effect on bone mineral metabolism after menopause, according to the biochemical results obtained (with markers of bone remodeling, vitamin D and PTH) and the densitometric (with DXA and QUS). Finally, the propensity score matching method allowed us to confirm that it did not influence the prevalence of fragility fractures after menopause either.
Bibliografía
1 Siris ES, Adler R, Bilezikian J, Bolognese M, Dawson-Hughes B, Favus MJ, et al. The clinical diagnosis of osteoporosis: A position statement from the National Bone Health Alliance Working Group. Osteoporos Int. 2014;25(5):1439-1443. [ Links ]
2 Compston JE, McClung MR, Leslie WD. Osteoporosis. Lancet. 2019;393(10169): 364-376. [ Links ]
3 Sosa-Henríquez M, Segarra-Sánchez MC, Limiñana-Cañal JM, Hernández-Hernández D, González-Pacheco A, Betancor-León P. Morbilidad y mortalidad de la fractura osteoporótica de la extremidad proximal del fémur tras un año de seguimiento. Med Clin. 1993;101(13):481-483. [ Links ]
4 Navarro MC, Sosa M, Saavedra P, Lainez P, Marrero M, Torres M, et al. Poverty is a risk factor for osteoporotic fractures. Osteoporos Int. 2009;20(3):393-398. [ Links ]
5 Navarro MDC, Saavedra P, Jódar E, Gómez De Tejada MJ, Mirallave A, Sosa M. Osteoporosis and metabolic syndrome according to socio-economic status, contribution of PTH, vitamin D and body weight: The Canarian osteoporosis poverty study (COPS). Clin Endocrinol (Oxf). 2013;78(5):681-686. [ Links ]
6 Gómez-De-Tejada Romero MJ, Navarro Rodríguez MDC, Saavedra Santana P, Quesada Gómez JM, Jódar Gimeno E, Sosa Henríquez M. Prevalence of osteoporosis, vertebral fractures and hypovitaminosis D in postmenopausal women living in a rural environment. Maturitas. 2014;77(3):282-286. [ Links ]
7 Stattin K, Michaelsson K, Larsson SC, Wolk A, Byberg L. Leisure-time physical activity and risk of fracture: a cohort study of 66,940 men and women. J Bone Miner Res. 2017;32(8):1599-1606. [ Links ]
8 Trajanoska K, Rivadeneira F. The genetic architecture of osteoporosis and fracture risk. Bone [Internet]. 2019;126:2-10. [ Links ]
9 Johansson H, Kanis JA, Odén A, McCloskey E, Chapurlat RD, Christiansen C, et al. A meta-analysis of the association of fracture risk and body mass index in women. J Bone Miner Res. 2014;29(1):223-233. [ Links ]
10 De Luis Román DA, Aller R, Perez Castri-llon JL, De Luis J, Gonzalez Sagrado M, Izaola O, et al. Effects of dietary intake and life style on bone density in patients with diabetes mellitus type 2. Ann Nutr Metab. 2004;48(3):141-145. [ Links ]
11 Marsh AG, Sánchez TV, Michelsen O, Chaffee F, Fagal SM. Vegetarian lifestyle and bone mineral density. Am J Clin Nutr. 1988;48:837-841. [ Links ]
12 Naves M, Díaz-López JB, Gómez C, Rodríguez-Rebollar A, Cannata-Andía JB. Determinants of incidence of osteoporotic fractures in the female Spanish population older than 50. Osteoporos Int. 2005;16(12):2013-2017. [ Links ]
13 Rea MF. Os benefícios da amamentação para a saúde da mulher. J Pediatr (Rio J). 2004;80(5):142-146. [ Links ]
14 Schnatz PF, Marakovits KA, O'Sullivan DM. Assessment of postmenopausal women and significant risk factors for osteoporosis. Obstet Gynecol Surv. 2010;65(9):591-596. [ Links ]
15 Wang Q, Huang Q, Zeng Y, Liang JJ, Liu SY, Gu X, et al. Parity and osteoporotic fracture risk in postmenopausal women: a dose-response meta-analysis of prospective studies. Osteoporos Int. 2016;27(1):319-330. [ Links ]
16 Song SY, Kim Y, Park H, Kim YJ, Kang W, Kim EY. Effect of parity on bone mineral density: A systematic review and metaanalysis. Bone. 2017;101:70-76. [ Links ]
17 Ozdemir F, Demirbag D, Rodoplu M. Reproductive factors affecting the bone mineral density in postmenopausal women. Tohoku J Exp Med. 2005;205(3):277-285. [ Links ]
18 Gumming RG, Klineberg RJ. Breastfeeding and other reproductive factors and the risk of hip fractures in elderly women. Int J Epidemiol. 1993;22(4):684-691. [ Links ]
19 Bolzetta F, Veronese N, De Rui M, Berton L, Carraro S, Pizzato S, et al. Duration of breastfeeding as a risk factor for vertebral fractures. Bone [Internet]. 2014;68:41-45. [ Links ]
20 Kim HJ, Kwon H, Oh SW, Lee CM, Joh HK, Kim Y, et al. Breast feeding is associated with postmenopausal bone loss: Findings from the Korea national health and nutrition examination survey. Korean J Fam Med. 2015;36(5):216-220. [ Links ]
21 Yan G, Huang Y, Cao H, Wu J, Jiang N, Cao X. Association of breastfeeding and postmenopausal osteoporosis in Chinese women: a community-based retrospective study. BMC Womens Health. 2019;19(1):1-7. [ Links ]
22 Ramalho AC, Lazaretti-Castro M, Houache O, Vieira JG, Cafalli F, Tavares F. Osteoporotic fractures of proximal femur: clinical and epidemiological features in a population of the city of Sao Paulo. Sao Paulo Med J. 2001;119(2):48-53. [ Links ]
23 Crandall CJ, Liu J, Cauley J, Newcomb PA, Manson JAE, Vitolins MZ, et al. Associations of Parity, Breastfeeding, and Fractures in the Women's Health Observational Study. Obs Gynecol. 2017;130(1):171-180. [ Links ]
24 Miyamoto T, Miyakoshi K, Sato Y, Kasuga Y, Ikenoue S, Miyamoto K, et al. Changes in bone metabolic profile associated with pregnancy or lactation. Sci Rep. 2019;9 (1):1-13. [ Links ]
25 Sosa M, Saavedra P, Del Pino-Montes J, Alegre J, Pérez-Cano R, Martínez Díaz Guerra G, et al. Postmenopausal women with Colles' fracture have lower values of bone mineral density than controls as measured by quantitative ultrasound and densitometry. J Clin Densitom. 2005;8(4):430-435. [ Links ]
26 Del Carmen Navarro M, Saavedra P, Gómez-de-Tejada MJ, Suárez M, Hernández D, Sosa M. Discriminative ability of heel quantitative ultrasound in postmenopausal women with prevalent vertebral fractures: Application of optimal threshold cutoff values using classification and regression tree models. Calcif Tissue Int. 2012;91(2):114-120. [ Links ]
27 Sosa M, Saavedra P, Muñoz-Torres M, Alegre J, Gómez C, González-Macías J, et al. Quantitative ultrasound calcaneus measurements: Normative data and precision in the Spanish population. Osteoporos Int. 2002;13(6):487-492. [ Links ]
28 Sosa M, Hernández D, Estévez S, Rodríguez M, Limiñana JM, Saavedra P, et al. The range of bone mineral density in healthy canarian women by dual X-ray absorptiometry radiography and quantitative computer tomography. J Clin Densitom. 1998;4:385-393. [ Links ]
29 World Medical Association. World Medical Association Declaration of Helsinki. Ethical Principles for Medical Research Involving Human Subjects. JAMA. 2013; 310(20):2013-2016. [ Links ]
30 NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285(6): 785-795. [ Links ]
31 Xiao H, Zhou Q, Niu G, Han G, Zhang Z, Zhang Q, et al. Association between breastfeeding and osteoporotic hip fracture in women: A dose-response meta-analysis. J Orthop Surg Res. 2020;15(1):1-7. [ Links ]
32 Kovacs CS. The skeleton is a storehouse of mineral that is plundered during lactation and (fully?) replenished afterwards. J Bone Miner Res. 2017;32(4):676-680. [ Links ]
33 Grizzo FMF, Alarcão ACJ, Dell' Agnolo CM, Pedroso RB, Santos TS, Vissoci JRN, et al. How does women's bone health recover after lactation? A systematic review and meta-analysis. Osteoporos Int. 2020;31 (3):413-427. [ Links ]
34 Lee EN. Effects of parity and breastfeeding duration on bone density in postmenopausal women. Asian Nurs Res (Korean Soc Nurs Sci) [Internet]. 2019;13(2):161-167. [ Links ]
35 Karlsson MK, Ahlborg HG, Karlsson C. Maternity and bone mineral density. Acta Or-thop Scand. 2005;76(1):2-13. [ Links ]
36 Duan X, Wang J, Jiang X. A meta-analysis of breastfeeding and osteoporotic fracture risk in the females. Osteoporos Int. 2017;28(2):495-503. [ Links ]
37 Bjørnerem Å, Ahmed LA, Jørgensen L, Størmer J, Joakimsen RM. Breastfeeding protects against hip fracture in postmenopausal women: The Tromsø study. J Bone Miner Res. 2011;26(12):2843-2850. [ Links ]
38 Wallach S, Feinblatt JD, Carstens JH, Avioli LV. The bone "quality” problem. Calcif Tissue Int. 1992;51(3):169-172. [ Links ]
39 Carneiro RM, Prebehalla L, Tedesco MB, Sereika SM, Hugo M, Hollis BW, et al. Lactation and bone turnover: A conundrum of marked bone loss in the setting of coupled bone turnover. J Clin Endocrinol Metab. 2010;95(4):1767-1776. [ Links ]
40 Sowers M. Pregnancy and lactation as risk factors for subsequent bone loss and osteoporosis. J Bone Miner Res. 1996;11(8): 1052-1060. [ Links ]
41 Yoo JE, Shin DW, Han K, Kim D, Yoon JW, Lee DY. Association of female reproductive factors with incidence of fracture among postmenopausal women in Korea. JAMA Netw Open. 2021;4(1):e2030405. [ Links ]
42 Canal-Macias ML, Roncero-Martin R, Moran JM, Lavado-Garcia JM, Costa-Fer-nandez MDC, Pedrera-Zamorano JD. Increased bone mineral density is associated with breastfeeding history in premenopausal Spanish women. Arch Med Sci. 2013;9(4):703-708. [ Links ]
43 Yang L, Waldhoer T. Statistically significant but clinically irrelevant correlation? Wien Klin Wochenschr. 2020;132(17-18): 547-548. [ Links ]
Received: April 01, 2021; Accepted: May 22, 2021











 texto em
texto em