Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista de Osteoporosis y Metabolismo Mineral
versão On-line ISSN 2173-2345versão impressa ISSN 1889-836X
Rev Osteoporos Metab Miner vol.15 no.3 Madrid Jul./Set. 2023 Epub 08-Mar-2024
https://dx.doi.org/10.20960/revosteoporosmetabminer.00019
ORIGINALS
Association of gamma glutamyl transferase in the presence and progression of abdominal aortic calcifications and changes to bone mineral density
1Clinical Management Unit of Bone Metabolism. Hospital Universitario Central de Asturias. Universidad de Oviedo. Instituto de Investigación Sanitaria del Principado de Asturias (ISPA). Oviedo, Spain
2Clinical Management Area of Nephrology. Hospital Universitario Central de Asturias. Universidad de Oviedo. Instituto de Investigación Sanitaria del Principado de Asturias (ISPA). Oviedo, Spain
4Laboratory of Medicine. Hospital Universitario Central de Asturias. Oviedo, Spain
5Clinical Management Unit of Internal Medicine. Hospital Universitario Central de Asturias. Universidad de Oviedo. ISPA. Oviedo, Spain
6Department of Functional Biology. Investigación Básica y Traslacional en Enfermedades inflamatorias Crónicas. Universidad de Oviedo. ISPA. Oviedo, Spain
Introduction and objective:
abdominal aortic calcification (AAC) is a predictor of cardiovascular events. This study aimed to assess the association of gamma glutamyl transferase (GGT) in the presence and progression of AAC, as well as changes to bone mineral density (BMD) in the lumbar spine and femoral neck.
Materials and methods:
a total of 326 men and women over 50 years of age were selected for this study. They completed a questionnaire, underwent two lateral dorso-lumbar spine X-rays, and BMD measurements. The same tests and 1 analytical assessment were repeated after 4 years.
Results:
the presence and progression of AAC (new occurrences or increased severity) were lower in GGT quartile 1 (Q1) compared with the other quartiles (40 % vs 58 %; p = 0.021; 24 % vs 44 %; p = 0.022). Compared with Q1, the confounders-adjusted logistic regression analysis showed that Q2 and Q4 were associated with more presence of AAC [odds ratio (OR), 2.53; 95 % confidence interval (95 % CI), 1.22-5.25 and OR, 3.04; 95 % CI, 1.36-6.77]. Additionally, Q2, Q3, and Q4 were associated with more AAC progression [OR, 2.24; 95 % CI, 1.07-4.67; OR, 2.35; 95 % CI, 1.09-5.07; and OR, 3.47; 95 % CI, 1.56-7.70]. The gender-stratified multivariate analysis revealed that in both men and women, the Q4 of GGT was associated with AAC progression [OR, 3.27; 95 % CI, 1.14-9.36, and OR, 3.26; 95 % CI, 1.03-10.29, respectively], and in women alone, with greater lumbar BMD losses. There were no effects regarding the prevalence of AAC.
Conclusions:
elevated GGT levels could serve as an indicator of the presence and progression of AAC in individuals older than 50 years. When analyzed separately by gender, higher GGT levels were associated with AAC progression, which acted as a prognostic marker for cardiovascular disease.
Keywords: Gamma glutamyl transferase; Abdominal aortic calcification; Overall population; Bone mineral density
INTRODUCTION
Vascular calcification is a process wherein vascular smooth muscle cells dedifferentiate into osteoblasts, resulting in the formation of bone in inappropriate locations. Vascular calcification is a significant issue of public health that is projected to escalate due to the aging population. Data from the European study on vertebral osteoporosis estimated a prevalence of abdominal aortic calcification in the overall population over 50 years old of 38 %, being more common in men (46 %) than women (30 %) (1). The abdominal aorta is one of the first vascular beds where atherosclerotic calcification can be seen, often preceding the development of coronary artery calcification (2,3). Abdominal aortic calcification contributes to arterial stiffness and strongly predicts cardiovascular events and mortality (4).
Numerous classical factors have been associated with abdominal aortic calcification, including age, hypertension, smoking, hyperlipidemia, diabetes mellitus, and overweight, among others. However, using one single biomarker to assess the presence of abdominal aortic calcification before its consequences start to show is still challenging. Gamma glutamyl transferase (GGT), an enzyme used as a marker of hepatobiliary diseases and alcohol consumption, is also recognized as a true marker of atherosclerotic disease. Former studies have demonstrated that serum GGT levels, even within normal ranges, are associated with atherosclerotic risk factors and predict the future occurrence of cardiovascular diseases, hypertension, stroke, metabolic syndrome, and type 2 diabetes (5-8).
A meta-analysis proved the existence of a relationship between GGT and the incidence of cardiovascular events regardless of alcohol consumption (9). While various studies have explored coronary aortic calcification and serum GGT levels in cross-sectional cohorts (10-13), we still need studies that other from confirming these findings in the abdominal aorta and within our geographic context, can prove that there is a potentially stronger association than the one found in the previously mentioned cross-sectional studies. Therefore, the objective of this study was to assess the predictive potential of GGT in the prevalence and progression of abdominal aortic calcification, and in changes to the lumbar spine and femoral neck bone mineral density (BMD) in an unselected overall population of men and women older than 50 years.
MATERIAL AND METHODS
This study was conducted using data from a European project aimed at determining the prevalence of vertebral fractures (European vertebral osteoporosis study - EVOS) (14) with participation from the Bone Metabolism Clinical Unit of Hospital Universitario Central de Asturias in Oviedo, Spain.
A random selection was made from the Oviedo municipal registry including 308 men and 316 women older than 50 years. The study protocol involved the completion of a questionnaire on osteoporosis-related risk factors, 2 lateral dorso-lumbar spine X-rays (the radiographic study was only incomplete in 2 cases), and the collection of anthropometric measurements such as height and weight to determine the body mass index (BMI). All participants had enough walking capabilities to climb 2 flights of stairs without an elevator, and 99 % of them lived in their own homes.
After 4 years, participants were invited to undergo the same radiographic study, anthropometric measurements, a questionnaire on osteoporosis risk factors, and a biochemical analysis. In the second follow-up, a total of 402 participants (213 women and 189 men) were included. The data of 326 subjects were available at the beginning of the study and 4 years later.
ASSESSMENT OF PROGRESSION OF VASCULAR CALCIFICATION
Abdominal aortic calcification was assessed by two independent investigators, defined, and categorized into 3 grades: grade 0 (absent), grade 1 (mild-to-moderate), and grade 2 (severe). Isolated punctate calcifications, a visible linear calcification spanning fewer than 2 vertebral bodies, or a dense calcified plaque were defined as mild-to-moderate calcification (1). The presence of a visible linear calcification extending across, at least, 2 vertebral bodies and/or the presence of 2 or more dense calcified plaques were categorized as severe calcification. The degree of intra- and inter-observer agreement for the radiographic analysis was 92 % and 90 %, respectively, with a Kappa coefficient of 0.78 and 0.73, indicative of good reproducibility (1).
The progression of aortic calcification was determined by comparing the X-rays taken at the beginning of the study with those taken 4 years later. Aortic calcification “progression” was defined as an increase in the extent of baseline aortic calcification along with the appearance of new calcifications, as seen in the comparison between the early x-rays and those taken 4 years later.
DENSITOMETRIC EVALUATION
BMD was measured using a Hologic® QDR-1000 DXA densitometer (Hologic Inc., Waltham, MA, United States). In all cases, measurements were taken of the antero-posterior lumbar spine (L2-L4) and right femoral neck. Regarding lumbar BMD assessment, a total of 4 participants with significant degenerative arthritis were excluded. The coefficients of variation (CV) were 1.2 % and 1.9 % respectively (1). Precision and quality control were kept daily using a lumbar spine phantom that yielded a CV of 0.0 ± 0.1 %. In the fourth year, BMD of the same areas was determined as in the early study, and the percentage change between the 2 measurements was used to assess changes to BMD.
BIOCHEMICAL ANALYSIS
During the baseline study, no biochemical analysis was ever conducted. After 4 years, fasting blood and urine samples were collected from each study participant. Once serum and urine samples were separated, they were frozen at -80 °C until quantification. Calcium, creatinine, phosphorus, total alkaline phosphatase, gamma glutamyl transferase (GGT), and serum tartrate-resistant acid phosphatase levels were measured using an autoanalyzer (Hitachi Mod. 717, Ratigen, Germany). The serum levels of calcidiol (25OHD) were determined through prior extraction with acetonitrile (IDS, Ltd., Bolton, United Kingdom), with intra- and inter-assay coefficients of variation (CV) of 5.2 % and 8.2 % respectively.
The levels of 1,25-dihydroxyvitamin D were measured using a radioimmunoassay (IDS, Ltd.), with intra- and inter-assay CVs of 6.5 % and 9 % respectively. Intact PTH and osteocalcin levels were measured using a radioimmunoassay (Nichols Institute, San Juan Capistrano, CA, United States). The intra- and inter-assay CVs were 2.6 % and 5.8 % for PTH, and 4.5 % and 5.1 % for osteocalcin, respectively.
All the studies conducted observed the principles outlined in the Declaration of Helsinki and were formally approved by the Clinical Trials Committee of the Principado de Asturias, Spain.
STATISTICAL ANALYSIS
Data analysis was conducted using version 25.0 of SPSS for Windows. Quantitative variables were analyzed using the Student t test for normally distributed variables and the Mann-Whitney U test for those following abnormal distributions. Qualitative variables were analyzed using the chi-square test.
Multivariate analysis of GGT quartiles was performed on the presence of abdominal aortic calcification, and with progression and/or appearance of new abdominal aortic calcification using logistic regression adjusted for age, sex, BMI, smoking habit, and alcohol intake.
Similarly, a multivariate analysis was performed for the highest GGT levels (quartile 4, while the remaining 3 quartiles were grouped in 1) associated with changes to BMD using linear logistic regression adjusted for age, sex, BMI, smoking habit, and alcohol intake.
RESULTS
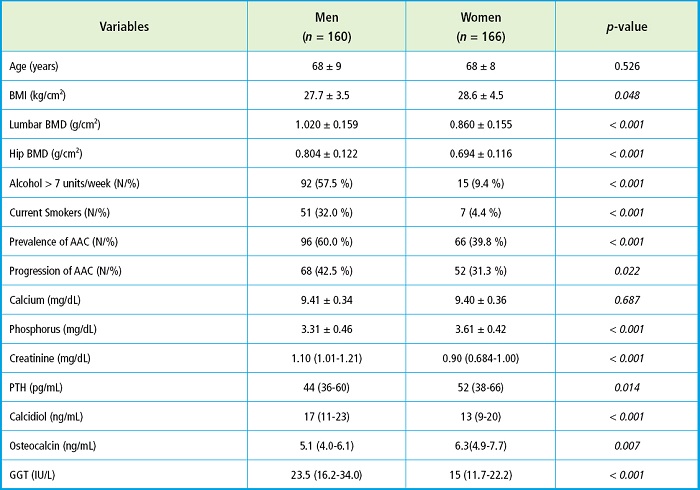
Table I shows the clinical characteristics, anthropometric data, and biochemical values differentiated by gender. The mean age was similar (68 years) with higher BMI reported in women. In men, BMD at the lumbar spine and femoral neck, the smoking habit, and weekly alcohol intake > 7 units of alcohol was significantly higher compared with women, as well as the prevalence and progression of abdominal aortic calcification. Among the biochemical parameters, men exhibited more elevated levels of serum creatinine, calcidiol, and GGT. Conversely, women had higher serum levels of phosphorus, PTH, and osteocalcin.
Table I. Clinical, anthropometric, and biochemical markers of bone and mineral metabolism between men and women.
To categorize any potential effect of GGT on abdominal aortic calcification, serum GGT levels were categorized into quartiles. Overall, the presence and progression of abdominal aortic calcification (new AAC or increased severity compared to baseline) were significantly lower in the lowest GGT quartile compared with the remaining quartiles (40 % vs 58 %; p = 0.021; 24 % vs 44 %, p = 0.022) (Table II). Compared with the lowest GGT quartile (reference), the logistic regression analysis adjusted for age, BMI, sex, smoking habit, and alcohol intake showed that Q2 and Q4 were associated with the presence of more abdominal aortic calcification [OR, 2.53; 95 % confidence interval (95 % CI), 1.22-5.25], and OR, 3.04; 95 % CI, 1.36-6.77, and that Q2, Q3, and Q4 were associated with more abdominal aortic calcification progression [OR, 2.24; 95 % CI, 1.07-4.67; OR, 2.35; 95 % CI, 1.09-5.07; and OR, 3.47; 95 % CI, 1.56-7.70].
Table II. Prevalence and progression of abdominal aortic calcification based on the levels of GGT in quartiles in the overall population.

Each quartile represents the number and percentage of individuals with prevalence and progression of AAC.
Considering the potential effect of alcohol consumption on the GGT levels, associations were analyzed separately based on gender. In the univariate analysis, there was no clear trend in the prevalence and progression of abdominal aortic calcification based on GGT quartiles (Table III). However, the logistic regression analysis conducted separately by gender and adjusted for age, BMI, smoking habit, and alcohol intake, revealed that in both men and women, higher GGT values (Q4) were associated with the progression of abdominal aortic calcification [OR, 3.27; 95 % CI, 1.14-9.36] and OR, 3.26; 95 % CI, 1.03-10.29, respectively. There was no effect reported on the prevalence of abdominal aortic calcification.
Table III. Prevalence and progression of abdominal aortic calcification based on the levels of GGT in quartiles separated by gender.

Each quartile represents the number and percentage of individuals with prevalence and progression of AAC.
Since the levels of GGT were independently associated with the progression of abdominal aortic calcification in both men and women and considering the association between the process of calcification and bone demineralization, changes to BMD between the 2 visits were studied. It was seen that, at lumbar level, and only in women, higher serum GGT levels (Q4) were associated with greater BMD losses compared to the remaining 3 GGT quartiles grouped together (Table IV). The linear regression analysis adjusted for age, BMI, smoking habit, and alcohol intake showed that the quartile with the highest GGT serum levels was significantly associated with changes to lumbar BMD (standardized beta coefficient of 0.245; p = 0.004). There were no significant differences found in women’s femoral neck, although the trend was similar (p = 0.090). In men, no effects were seen in the 2 bone segments analyzed (Table IV). With the remaining bone segments available (trochanter, total hip, or Ward’s triangle), no associations with serum GGT levels were found in men or women (data not available).
DISCUSSION
The outcomes of this study reveal a clear association between serum GGT levels and abdominal aortic calcification in a cohort of men and women with a mean age of 68 years, as former studies on coronary aortic calcification had already confirmed (10-13). Gender-specific analysis proved that in both men and women, the highest GGT quartile was associated with more than a 3-fold increase in the progression of calcification, including new calcifications and the worsening severity of the already existing ones. Other authors have also noted that GGT is independently associated with coronary aortic calcification only in women, not in men (11). However, some authors found this association in both genders (10,13) or only in men (12). All these works come from cross-sectional, unlike our study involving longitudinal tracking of abdominal aortic calcification progression.
Atherosclerosis could be a precursor to vascular calcification, and the association between GGT and atherosclerosis is well-established. However, the exact mechanisms remain to be elucidated (15). Several mechanisms have been proposed though. The first mechanism suggests that GGT is associated with multiple atherosclerotic risk factors. Former studies demonstrated that serum GGT levels were associated with hypertension, metabolic syndrome, and diabetes mellitus (8,16-18), and increase insulin resistance (19). The second mechanism proposed is that GGT serves as a biomarker of oxidative stress. Physiologically, GGT acts as a protein catalyst in the degradation of glutathione, the body’s primary thiol antioxidant, potentially making it a proatherogenic marker (20). The third mechanism proposed is subclinical chronic inflammation. Former studies showed that there was a relationship between GGT levels and the levels of C-reactive protein (CRP) (21,22). Inflammation is known to be a crucial mechanism in all stages of cardiovascular disease (23). GGT mediates the interconversion of the inflammatory mediator leukotriene C4 containing glutathione into leukotriene D4 (24). The fourth mechanism proposed is the straightforward atherogenic potential of GGT. Some studies have identified enzymatically active GGT in coronary and carotid atheromas at the time of surgical atherectomy (25,26). It has been suggested that GGT may participate in disease progression by modulating one or more redox-sensitive processes involved in atherosclerosis (20,25).
Not only the association between serum GGT levels and coronary aortic calcification has been established, but also several studies have also associated serum GGT levels to valvular calcification (27,28). The role of GGT in tissue calcium balance was already studied by Niida et al. (29) who proved that the overexpression of GGT in transgenic mice accelerated bone resorption, leading to osteoporosis, possibly by stimulating the receptor activator of nuclear factor kappa-B ligand (RANKL) (30). In our study, higher GGT values in women were associated with greater bone loss at lumbar spine level, with an obvious although non-significant trend reported at femoral neck level. Perhaps focusing on aortic calcifications in the vicinity of the spine contributed to the lack of a relationship with another bone segment like the hip assuming that the theory of greater bone demineralization leading to greater calcification is correct (1). Femoral calcification should have been determined here to see if it was associated with changes to femoral BMD. In men, no effects were seen, probably due to women’s greater tendency towards osteoporosis.
This study has several limitations. Firstly, the serum determination of GGT was only conducted in the second cross-sectional analysis, thus limiting the associations found. Secondly, another potential limitation is that aortic calcification might overlap or juxtapose with lumbar vertebrae, leading to increased BMD values not due to actual changes to bone mass. However, this fact would not limit but rather reinforce our results as the overlay of calcifications might increase but not decrease BMD, thus reducing the real association between vascular calcification progression and BMD decrease like some studies suggest (1). Furthermore, the evaluation of vascular calcification was conducted on a plain x-ray and more sensitive imaging modalities were never used. It is also possible that some of the individuals who attended the second follow-up at 4 years would’ve done so due to worse physical condition compared to those who didn’t attend, although no clear selection biases were ever reported (31).
Despite these limitations, the study also possesses significant strengths, such as the substantial response rate of participants both at baseline (50 %) (32) and at the 4-year follow-up (70 %). The degree of inter-observer reproducibility to assess vascular calcification supports its use as a diagnostic criterion. Finally, unlike other studies, this one was prospective rather than cross-sectional, as most referenced studies are. This reinforces the validity of the findings, as well as their stronger degree of association.
In conclusion, we can assert that elevated GGT levels were associated with the presence and progression of abdominal aortic calcification, and in women, with greater lumbar BMD loss. When analyzed by gender, higher GGT values were associated with the progression of abdominal aortic calcification, thus confirming the utility of this marker as a cardiovascular risk factor regardless of gender and alcohol consumption.
ACKNOWLEDGEMENTS
This study has received partial funding from the European vertebral osteoporosis study (EVOS), E.U. (1991-1993); the European prospective osteoporosis study (EPOS), E.U. (BIOMED 93-95), the BMHI-CT 092-0182 (1993-1997); the Health Research Fund (FIS 94/1901-E); the Network of Renal Research (REDinREN) of ISCIII (RD06/0016/1013, RD12/0021/0023, RD16/0009/0017, RICORS2040 - Kidney Disease); the National R & D & I Plan 2008-2011, State R & D & I Plan 2013-2016, the European Regional Development Fund (ERDF), the Science, Technology, and Innovation Plan 2013-2017 and 2018-2022 of the Principado de Asturias (GRUPIN14-028, IDI-2018-000152, IDI-2021-000080), and Fundación Renal Iñigo Álvarez de Toledo (FRIAT). Beatriz Martín Carro has been funded by a predoctoral Severo Ochoa contract from the Principado de Asturias, Spain.
REFERENCES
1. Naves M, Rodríguez-García M, Díaz-López JB, Gómez-Alonso C, Cannata-Andía JB. Progression of vascular calcifications is associated with greater bone loss and increased bone fractures. Osteoporos Int 2008;19(8):1161-6. DOI: 10.1007/s00198-007-0539-1 [ Links ]
2. Strong JP, Malcom GT, McMahan CA, Tracy RE, Newman WP III, Herderick EE, et al. Prevalence and extent of atherosclerosis in adolescents and young adults: implications for prevention from the pathobiological determinants of atherosclerosis in youth study. JAMA 1999;281:727-35. DOI: 10.1001/jama.281.8.727 [ Links ]
3. Allam AHA, Thompson RC, Eskander MA, Mandour Ali MA, Sadek A, Rowan CJ, et al. Is coronary calcium scoring too late? Total body arterial calcium burden in patients without known CAD and normal MPI. J Nucl Cardiol 2018;25:1990-8. DOI: 10.1007/s12350-017-0925-9 [ Links ]
4. Bartstra JW, Mali WP, Spiering W, de Jong PA. Abdominal aortic calcification: from ancient friend to modern foe. Eur J Prev Cardiol 2021;28(12):1386-91. DOI: 10.1177/2047487320919895 [ Links ]
5. Emdin M, Passino C, Michelassi C, Donato L, Pompella A, Paolicchi A. Additive prognostic value of gamma-glutamyltransferase in coronary artery disease. Int J Cardiol 2009;136:80-5. DOI: 10.1016/j.ijcard.2008.04.030 [ Links ]
6. Lee DH, Jacobs Jr DR, Gross M, Kiefe CI, Roseman J, Lewis CE, et al. Gamma-glutamyltransferase is a predictor of incident diabetes and hypertension:the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Clin Chem 2002;49:1358-66. DOI: 10.1373/49.8.1358 [ Links ]
7. Perry IJ, Wannamethee SG, Shaper AG. Prospective study of serum gammaglutamyltransferase and risk of NIDDM. Diabetes Care 1998;21:732-7. DOI: 10.2337/diacare.21.5.732 [ Links ]
8. Bozbaş H, Yıldırır A, Karaçağlar E, Demir O, Ulus T, Eroğlu S, et al. Increased serum gammaglutamyltransferase activity in patients with metabolic syndrome. Turk Kardiyol Dern Ars 2011;39:122-8. DOI: 10.5543/tkda.2011.01205 [ Links ]
9. Fraser A, Harris R, Sattar N, Ebrahim S, Smith GD, Lawlor DA. Gamma-glutamyltransferase is associated with incident vascular events independently of alcohol intake:analysis of the British Women's Heart and Health Study and Meta-Analysis. Arterioscler Thromb Vasc Biol 2007;27:2729-35. DOI: 10.1161/ATVBAHA.107.152298 [ Links ]
10. Atar AI, Yilmaz OC, Akin K, Selcoki Y, Er O, Eryonucu B. Association between gamma-glutamyltransferase and coronary artery calcification. Int J Cardiol 2013;167:1264-7. DOI: 10.1016/j.ijcard.2012.03.157 [ Links ]
11. Bian LQ, Zhangb ZY, Kim SJ, Zhoue CC, Choif YH. Gamma glutamyltransferase as a novel marker of coronary artery calcification in women. J Cardiovasc Med 2012;13:684-90. DOI: 10.2459/JCM.0b013e328356a432 [ Links ]
12. Cho HS, Lee SW, Kim ES, Mo EY, Shin JY, Moon SD, et al. Clinical significance of serum bilirubin and cgammaglutamyltransferase levels on coronary atherosclerosis assessed by multidetector computed tomography. Nutr Metab Cardiovasc Dis 2015;25(7):677-85. DOI: 10.1016/j.numecd.2015.03.014 [ Links ]
13. Lee W, Ryoo JH, Suh BS, Lee J, Kim J. Association of coronary artery calcification and serum gamma-glutamyl transferase in Korean. Atherosclerosis 2013;226(1):269-74. DOI: 10.1016/j.atherosclerosis.2012.10.059 [ Links ]
14. Neill TW, Felsenberg D, Varlow J, Cooper C, Kanis JA, Silman AJ. The prevalence of vertebral deformity in european men and women: the European Vertebral Osteoporosis Study. J Bone Miner Res 1996;11(7):1010-8. DOI: 10.1002/jbmr.5650110719 [ Links ]
15. Ndrepepa G, Colleran R, Kastrati A. Gamma-glutamyl transferase and the risk of atherosclerosis and coronary heart disease. Clin Chim Acta 2018;476:130-8. DOI: 10.1016/j.cca.2017.11.026 [ Links ]
16. Emdin M, Passino C, Michelassi C, Donato L, Pompella A, Paolicchi A. Additive prognostic value of gamma-glutamyltransferase in coronary artery disease. Int J Cardiol 2009;136:80-5. DOI: 10.1016/j.ijcard.2008.04.030 [ Links ]
17. Lee DH, Jacobs Jr DR, Gross M, Kiefe CI, Roseman J, Lewis CE, et al. Gamma-glutamyltransferase is a predictor of incident diabetes and hypertension:the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Clin Chem 2003;49:1358-366. DOI: 10.1373/49.8.1358 [ Links ]
18. Perry IJ, Wannamethee SG, Shaper AG. Prospective study of serum gammaglutamyltransferase and risk of NIDDM. Diabetes Care 1998;21:732-7. DOI: 10.2337/diacare.21.5.732 [ Links ]
19. Bonnet F, Ducluzeau PH, Gastaldelli A, Laville M, Anderwald CH, Konrad T, et al. Liver enzymes are associated with hepatic insulin resistance, insulin secretion, and glucagon concentration in healthy men and women. Diabetes 2011;60:1660-7. DOI: 10.2337/db10-1806 [ Links ]
20. EmdinM, Pompella A, Paolicchi A. Gamma-glutamyltransferase, atherosclerosis, and cardiovascular disease: triggering oxidative stress within the plaque. Circulation 2005;112:2078-80. DOI: 10.1161/CIRCULATIONAHA.105.571919 [ Links ]
21. Bo S, Gambino R, Durazzo M, Guidi S, Tiozzo E, Ghione F, et al. Associations between gamma-glutamyl transferase, metabolic abnormalities and inflammation in healthy subjects from a population-based cohort: a possible implication for oxidative stress. World J Gastroenterol 2005;11(45):7109-17. DOI: 10.3748/wjg.v11.i45.7109 [ Links ]
22. Lee DH, Jacobs Jr DR. Association between serum gamma-glutamyltransferase and C-reactive protein. Atherosclerosis 2005;178:327-330. DOI: 10.1016/j.atherosclerosis.2004.08.027 [ Links ]
23. Targher G, Seidell JC, Tonoli M, Muggeo M, De Sandre Gr cardiovascular risk factors in healthy male individuals. J Intern Med 1996;239:435-41. DOI: 10.1046/j.1365-2796.1996.815000.x [ Links ]
24. Anderson ME, Allison RD, Meister A. Interconversion of leukotrienes catalyzed by purified gamma-glutamyl transpeptidase: concomitant formation of leukotriene D4 and gamma-glutamyl amino acids. Proc Natl Acad Sci USA 1982;79:1088-91. DOI: 10.1073/pnas.79.4.1088 [ Links ]
25. Paolicchi A, Emdin M, Ghliozeni E, Ciancia E, Passino C, Popoff G, et al. Images in cardiovascular medicine. Human atherosclerotic plaques contain gamma-glutamyl transpeptidase enzyme activity. Circulation. 2004;109:1440. DOI: 10.1161/01.CIR.0000120558.41356.E6 [ Links ]
26. Franzini M, Corti A, Martinelli B, Del Corso A, Emdin M, Parenti GF, et al. Gamma-glutamyltransferase activity in human atherosclerotic plaques-biochemical similarities with the circulating enzyme. Atherosclerosis 2009;202:119-27. DOI: 10.1016/j.atherosclerosis.2008.03.023 [ Links ]
27. Bozbas H, Yildirir A, Demir O, Cakmak A, Karacaglar E, Yilmaz M, et al. Serum gamma-glutamyltransferase activity is increased in patients with calcific aortic valve stenosis. J Heart Valve Dis 2008;17(4):371-5. [ Links ]
28. Cappelli S, Epistolato MC, Vianello A, Cappelli S, Mazzone A, Glauber M, et al P. Aortic valve disease and gammaglutamyltransferase: accumulation in tissue and relationships with calcific degeneration. Atherosclerosis 2010;213(2):385-91. DOI: 10.1016/j.atherosclerosis.2010.08.063 [ Links ]
29. Niida S, Kawahara M, Ishizuka Y, Ikeda Y, Kondo T, Hibi T, et al. Gamma-glutamyltranspeptidase stimulates receptor activator of nuclear factor-kappaB ligand expression independent of its enzymatic activity and serves as a pathological bone-resorbing factor. J Biol Chem 2004;279:5752-6. DOI: 10.1074/jbc.M311905200 [ Links ]
30. Hiramatsu K, Asaba Y, Takeshita S, Nimura Y, Tatsumi S, Katagiri N, et al. Overexpression of gammaglutamyltransferase in transgenic mice accelerated bone resorption and causes osteoporosis. Endocrinology 2007;148:2708-15. DOI: 10.1210/en.2007-0215 [ Links ]
31. O'Neill TW, Marsden D, Silman AJ. Differences in the characteristics of responders and non-responders in a prevalence survey of vertebral osteoporosis. European Vertebral Osteoporosis Study Group. Osteoporos Int 1995;5(5):327-34. [ Links ]
32. Naves M, Díaz López JB, Virgós MJ, O'Neill TW, Gómez C, Zaplana J, et al. Índices de participación y aspectos metodológicos de interés en un estudio de prevalencia de fractura vertebral en Asturias. REEMO 1993;2(5):29-32. DOI: 10.1007/BF01622254 [ Links ]
Martín Carro B, Gómez Alonso C, Rodríguez García M, Avello Llano N, García Gil-Albert C, Sobrino Díaz L, Baena Huerta F, Palomo Antequera C, Naves Mendívil L, Rodríguez Carrio J, Fernández Martín JL, Naves Díaz M. Association of gamma glutamyl transferase in the presence and progression of abdominal aortic calcifications and changes to bone mineral density. Rev Osteoporos Metab Miner 2023;15(3):93-99
Funding:
this original manuscript has been funded with a FEIOMM 2022 travel grant on behalf of Beatriz Martín Carro.
This study has received partial funding from the European vertebral osteoporosis study (EVOS), E.U. (1991-1993); the European prospective osteoporosis study (EPOS), E.U. (BIOMED 93-95), the BMHI-CT 092-0182 (1993-1997); the Health Research Fund (FIS 94/1901-E); the Network of Renal Research (REDinREN) of ISCIII (RD06/0016/1013, RD12/0021/0023, RD16/0009/0017, RICORS2040 - Kidney Disease); the National R & D & I Plan 2008-2011, State R & D & I Plan 2013-2016, the European Regional Development Fund (ERDF), the Science, Technology, and Innovation Plan 2013-2017 and 2018-2022 of the Principado de Asturias (GRUPIN14-028, IDI-2018-000152, IDI-2021- 000080), and Fundación Renal Iñigo Álvarez de Toledo (FRIAT). Beatriz Martín Carro has been funded by a predoctoral Severo Ochoa contract from the Principado de Asturias, Spain.
Received: June 01, 2023; Accepted: August 01, 2023











 texto em
texto em 



