INTRODUCTION
Osteoarthritis (OA) is currently recognized as a multifactorial disease in which various factors can generate and perpetuate damage to articular cartilage, with the subsequent response of the synovial membrane and subchondral bone. Knee OA has been regarded as a purely mechanical condition, with the emphasis on joint overloads associated with axis changes, traumatic injuries, and multi-ligament instabilities. When chondral extracellular matrix (ECM) is compromised, there is less water retention and the tissue loses resistance, resilience, and elasticity to compression, thereby increasing damage to the surrounding tissue. Due to the low rate of cell turnover and poor reparative capacity, the cartilage fails to compensate for the damage sustained (1,2). Regardless of the original cause of the damage, synovial membrane fibroblasts respond by secreting various cytokines and inflammatory factors (IL-1, TNF-α, TGF-β, IL-8, among others) (3,4). These inflammatory factors remain present in the joint, regardless of the corrective treatment of the original cause of chondral damage (ligament stabilization, fracture reduction, axe correction, etc.), being able to maintain the progression of the damage (1,2,5).
Currently, various conservative treatments are used to treat knee osteoarthrosis, including drug therapy with non-steroidal anti-inflammatory drugs (NSAIDs) and analgesics (AAS, paracetamol). Also, natural products, like glycosaminoglycans, chondroitin-sulfate, or collagen are advised. Intra-articular therapy involves the restoration of the usual biological properties, viscosity and elasticity, and synovial fluid using hyaluronic acid, which regulates various cellular activities and restores the properties of synovial fluid. On the other hand, platelet-rich plasma (PRP), that uses a high concentration of platelets (2 to 4 times higher), releases growth factors with chondrogenic properties and anti-inflammatory cytokines.
The objective of this study is to determine if an oral therapy based on 10 g of hydrolyzed collagen along with 100 mg fucoidan (Hydroidan Pro, Acten, Switzerland)—a sulfated polysaccharide that comes from some types of brown algae and has proven to help reduce inflammatory factors—effectively reduces symptoms of grade II-III gonarthrosis on the Kellgren and Lawrence scale compared with intra-articular hyaluronic acid, or intra-articular platelet-rich plasma.
MATERIALS AND METHODS
STUDY DESIGN AND ETHICAL ASPECTS
This was a prospective, longitudinal, analytical, randomized, and single-blind study to assess the efficacy profile of an oral therapy (10 g hydrolyzed collagen along with 100 mg fucoidan, Hydroidan Pro, Acten, Switzerland) compared with intra-articular treatments (hyaluronic acid and platelet-rich plasma) to treat knee osteoarthritis. A digital app for randomization (https://www.randomizer.com) was used to allocate patients to 3 different groups. Following the center review board approval (OR17-00016) patients were actively recruited in the Department of Orthopedics and Traumatology. All patients provided their prior written informed consent to participate in the study. This study was conducted in accordance with the World Medical Association Declaration of Helsinki. The resources and funding to conduct this study were provided by our hospital Department of Orthopedics and Traumatology.
PARTICIPANTS AND STUDY SUBJECTS
From October 2017 through November 2019, we invited all patients aged between 40 and 90 years with diagnosed knee osteoarthritis (based on the American College of Rheumatology criteria), a > 12-month history of symptoms, and grade II-III osteoarthritis in the Kellgren-Lawrence classification to join our study. Exclusion criteria included pregnant or breastfeeding woman, rheumatoid arthritis, knee surgery or arthroscopy, use of intra-articular steroids, hyaluronic acid, or platelet-rich plasma in the previous 9 months, cancer in the past 5 years, glucosamine and chondroitin therapy in the previous 6 months, smokers (20 or more packs of cigarettes/year), alcohol users (50 or more grams/week), comorbidities such as gout (uric acid of 6.8 mg/dL or more), chronic renal disease (GFR < 60 mL/min/1.73 m2), non-controlled diabetes mellitus (Hb1Ac > 7 %), non- controlled hypertension (> 120/> 80 or more), or patients who were participating in different studies. Exclusion criteria included a follow-up or missing a dose of the oral treatment.
During recruitment period from October 2017 through November 2019, a total of 301 patients were scheduled to evaluate the clinical and radiographic criteria. However, 190 patients were ineligible (78 patients, Hb1Ac > 7; 34 patients, BP > 120/> 80; 32 patients: Kellgren-Lawrence grade I or IV; 31 patients on steroid therapy 9 months prior; 10 patients on glucosamine or chondroitin therapy 6 months prior; 5 patients with a history of cancer in the past 5 years); and 3 patients refused to participate in the study. A total of 108 patients were included in the study in 3 groups: group 1: n = 36, group 2: n = 36, and group 3: n = 36. No patients were lost or excluded at the follow up.
No differences were observed for any demographic or clinical outcome variable.
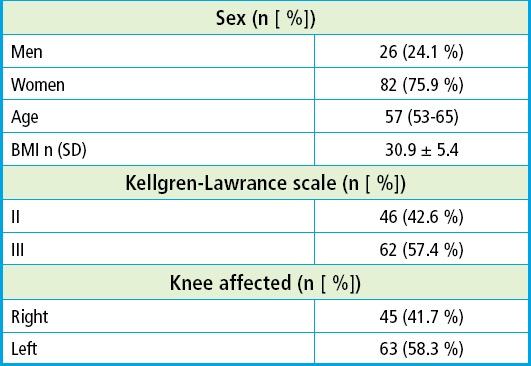
The baseline characteristics included age, biological sex, height, weight, BMI, Kellgren-Lawrence grade and knee. The baseline score of the three groups showed no statistically significant differences. The mean age was 57 (53-65) years. Twenty-six patients (24.1 %) were men and eighty-two (75.9 %) were women. The mean body mass index was 30.9 ± 5.4 kg/m2, being most patients ranked as grade I obesity. A total of 46 patients (42.6 %) were grade II according to the Kellgren-Lawrence scale while 62 (57.4 %) were grade III. A total of 45 (41.7 %) had more pain in their right knee and 63 (58.3 %) in their left knee (Table I and Fig. 1).
This study proposed a 1:1:1: randomization into the 3 groups. A correlative identification number was given after the informed consent was signed. The patients enrolled were assigned to one of three groups (group 1, collagen-fucoidan; group 2, hyaluronic acid, and group 3, platelet-rich plasma). Patients were randomized by a staff member from our hospital who wasn't engaged in this study.
Group 1 (hydrolyzed collagen plus 100 mg of fucoidan, Hydroidan Pro, Acten, Switzerland) received a single dose of a saline solution (5 mL) as placebo, and a 23 g dose of Hydroidan Pro orally, daily, for 24 weeks. Group 2 (hyaluronic acid) received a single dose of hyaluronic acid (5 mL) and a 23 g dose of chlorophyll as placebo. Group 3 (platelet-rich plasma) received a single dose of platelet-rich plasma (3 mL) and a 23 g dose of chlorophyll as placebo. Collagen and chlorophyll were changed to a metal-like plastic bag and the syringes of saline solution and hyaluronic acid or platelet-rich plasma were personally delivered to the doctor working on the knee infiltrations.
Infiltration technique
All infiltrations were performed by the same physician. Patients laid down in prone position. Asepsis and antisepsis were achieved with povidone iodine 8 %, then a sterile field was used to delimitate the working area. Patients were infiltrated with 3 mL of lidocaine (20 mg/mL) for anesthesia. The external suprapatellar technique was used in 102 patients (96.2 %) and the external subrotulian technique in 4 (3.7 %), which was left to the physician's criterion. After infiltration, the area was covered with a band-aid and the knee was flexed. Prophylactic antibiotics were not administered.
OUTCOME MEASUREMENTS
Demographic characteristics (age, biological sex, weight, height, and BMI), Kellgren-Lawrence classification, affected knee and comorbidities were addressed and collected. We used the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), the SF-12, and the VAS scale at baseline, and 4, 12 and 24 weeks later. The primary endpoint was the WOMAC scale at the 24-week follow-up compared to baseline. The secondary endpoints were the VAS and the SF-12 scale at the 24-week follow-up up compared to baseline. The WOMAC scale used was the 3.1 version (0-96). The SF-12 scale was the 2 version one (both bphysical and mental). The VAS scale was the linear one (0-10).
STATISTICAL ANALYSIS
The study sample size was calculated using an adjusted mean estimation formula in two populations, with an expected decrease of 60 ± 15 points for WOMAC in group 1 (Hydroidan Pro) vs 50 ± 10 points in the remaining therapies, with an 80 % statistical power and a two-tailed significance level of5 %. At least, a total of 29 patients were required per treatment group.
Statistical analysis was performed using IBM SPSS version 25 statistical package (Armonk, NY; IBM Corp.). The main characteristics of the population were described. Categorical variables were expressed as frequencies and percentages. The continuous ones as means ± standard deviation (SD) or median (interquartile range), after assessing the normality of data distribution using the Kolmogórov-Smirnov test. The intra-group comparisons of the different scores obtained throughout the different evaluations were performed using the Wilcoxon test. For intergroup comparisons, the deltas (Δ) of the differences of each measurement with respect to the baseline scores were calculated and compared using the Kruskal-Wallis's test. Post hoc analysis with Bonferroni correction was used to identify significant measurement or inter-group differences over time. p values < 0.05 were considered as statistically significant.
RESULTS
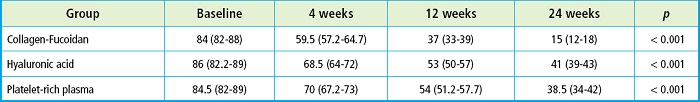
A comparison of the WOMAC score (at baseline, and 4, 12 and 24 weeks later) was made. In the three groups (Hydroidan Pro, HA ,and PRP) lower scores were seen at the 24-week follow-up, with a mean reduction from 84 down to 15 points in group 1 (Hydroidan Pro) (p < 0.001), 86 down to 41 points in group 2 (HA) (p < 0.001), and 84.5 down to 38.5 in group 3 (PRP) (p < 0.001) (Table II). After a post-hoc analysis, we found score differences on weeks 4, 12 and 24 compared with the baseline evaluation of the 3 lines (p < 0 .001).
Compared with the level of pain reported by the visual analogue scale, we found a significant reduction in the 3 groups, with a mean 8 to 1 points in group 1 (Hydroidan Pro) (p < 0.001), 8 to 2 in group 2 (HA) (p < 0.001) and 8 to 1.5 in group 3 (PRP) (p < 0.001) (Table III). After the post-hoc analysis, pain reduction was significantly less in each of the evaluations (on weeks 4, 12, and 24) compared with the baseline score (p < 0.05).
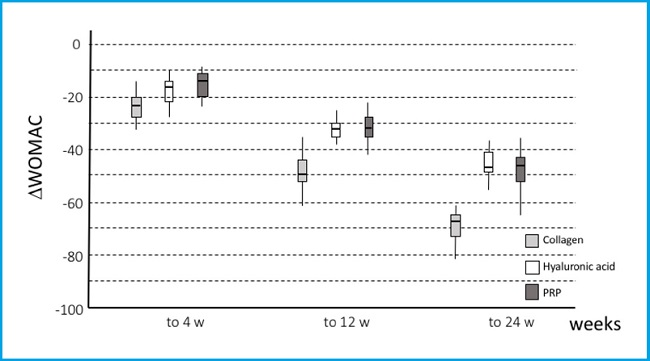
After the Δ of change in the WOMAC scale on the score's calculation on weeks 4, 12 and 24 weeks compared with baseline, we found a reduction of 23.5, 15.5, and 15 points on week 4; 50, 32.5, and 31 on week 12; and 68, 46, and 46.5 on week 24 in all groups (Hydroidan Pro, HA and PRP, respectively) (p < 0.001). Additionally, the Hydroidan Pro group showed minor WOMAC scores compared with patients treated with hyaluronic acid and platelet-rich plasma (p < 0 .001) (Fig. 2 and Table IV).
Table IV. WOMAC and VAS scores of the 3 groups at the 24-week follow-up compared with baseline values.

The lower pain reported with the VAS at the follow-up was significantly different among the groups, with lower scores being reported in patients treated with Hydroidan Pro compared with those treated with HA on weeks 4, 12, and 24 (p = 0.016, p < 0.002, and p < 0.001, respectively) and those treated with PRP on week 12 (p = 0.0031).
Also, we found lower scores in the PRP group compared with the HA group on week 12 (p = 0.031).
No adverse events occurred at the follow-up that were associated with the use of drugs. Three cases (2.7 %) of pain in the infiltration site for 3 or more days were reporte: 2 in the hyaluronic acid group and 1 in the Hydroidan Pro group.
DISCUSSION
The use of viscosupplementation in knee OA is extensive and fraught with heterogeneous trials with conflicting conclusions (6). There are two types of viscosupplementation hyaluronates and hylan. Hyaluronates are sodium hyaluronate and can be considered a drug as its mechanism of action is described mainly through a pharmacological mechanism that stimulates the endogenous synthesis of HA, which explains the extended duration of action. Hylan is considered an intra-articular implant since its mechanism of action is mainly through a physical mechanism. However, the mechanism of action of these products is not completely clear (7). By means of viscosupplementation, the production of IL-1 and other mediators of inflammation decreased. Likewise, the production of metalloproteases (MMP's) that degrade the articular cartilage decreased. In regard to the adverse events, although they have a good safety and tolerance profile, pain, swelling and effusion may occur in the infiltration area, known as temporary arthralgia, although some cases of pseudogout arthritis due to deposits of calcium pyrophosphate crystals have also been reported (7,8).
Within the biological treatment of osteoarthritis, PRP must be considered. PRP include a higher number of platelets than normal blood values. Platelets are enucleated cells traditionally characterized as main actors of the process of hemostasis, which is mediated by the release of proteins during its activation. PRP can be obtained and prepared from an individual's peripheral venous blood, through one or two subsequent centrifugation steps and with the use of basic laboratory materials or equipment (9). PRP is an effective intervention to treat knee OA without an increased risk of adverse events (10). The single administration of high volume pure PRP provided significant clinical benefit for 84.2 % of responders three months after the procedure. The KOOS total score significantly increased six months after the procedure, pain also significantly decreased and no difference was observed on MRI parameters (11). There are so many and such varied uses that detractors attribute this great variety of clinical applications and therapeutic benefits to a commercial benefit rather than a true effect on their “regenerative” capacity. The fact that platelets secrete growth factors and active metabolites leads us to believe that their use can have a positive influence in clinical cases that require rapid healing and tissue regeneration.
Advocates of this technique maintain that growth factors stimulate the synthesis of proteoglycans, aggrecans, and type II collagen by chondrocytes; induce synoviocyte proliferation, reduce catabolic effects of cytokines, such as interleukin-1 (IL-1) and MMP's, and the fact that it is an autologous preparation exempts it from harmful effects on joint tissues of patients (7,8). Among controversial issues regarding PRP, the lack of consensus on the exact composition stands out. Another frequent inconsistency is the composition of PRP and the heterogeneity of available techniques for its preparation. Several commercial presentations can vary significantly in the number of platelets, leukocytes, and erythrocytes. The initial centrifugation of the patient's blood separates red blood cells from plasma. Separated plasma may contain various concentrations of platelets with or without white blood cells. The platelets contained in this plasma can be further activated using thrombin, calcium chloride, calcium gluconate, freezing, and thawing, and this is subsequently applied to the area of injury as an infiltration while still liquid (12). This type of treatment can be conducted as a minimally invasive, outpatient treatment, providing a preparation directly to the area of injury with an immediate release of growth factors.
But for years the use of natural products that could “regenerate” cartilage has been defended. Among which are nutraceuticals, food products that have beneficial consequences for the body and can even act as drugs. Food products without any process or study must be distinguished from those found in nature or manipulated somehow. Collagen of animal origin is found in animal food and many collagen supplements are also sold. However, having them does not guarantee that it will be absorbed by the body. These supplements need to administered through a vehicle that allows its complete intestinal absorption so they can have an effect. In our case, collagen is added to a gel that allows the slow absorption of most of its collagen content. In addition, it is associated with another natural product, the extract of an algae, fucoidan, which is not a food, but has proven anti-inflammatory effects. Hence, there is a great variability of results in the different studies conducted, since not all collagens have the same quality or the same absorption capacity. As Deal and Moskowitz (13) put it, the name alone is not enough; you must know the doses, manufacturing and origin of the products that are indicated, because they're not all the same.
The intake of hydrolyzed collagen has been associated with pain relief and increased function in patients with OA. It has been suggested to use pharmaceutical grade hydrolyzed collagen as a modifying agent to treat OA based on the mechanisms of action of collagen as a tissue stimulant. Collagen as a nutritional supplement has been researched for the management of patients with OA and other types of joint pain. Experimental studies with bovine cartilage and cell cultures have shown that the administration of hydrolyzed collagen peptides increases type II collagen synthesis by chondrocytes (14). Moskowitz et al. (12) treated 52 patients of 56 years of age, with four treatments, three collagen preparations and egg albumin as control. During the study, patients were allowed to continue to use the analgesics or anti-inflammatory agents that they used to treat their symptoms before the study, maintaining a stable dose throughout their participation. All three collagen preparations were significantly superior to egg albumin in reducing pain compared to baseline. Side effects included mainly “an uncomfortable heaviness in the stomach”. At the end of the test cycle with any of the collagen-containing preparations, analgesic consumption was significantly reduced compared to consumption before treatment, in contrast to the control group. No radiographic changes were seen during the study period. Lab test results indicated no changes in liver function studies or antibody titers in the 3 types of collagens studied (1). The authors suggested that collagen has a direct analgesic effect or that collagen administration provides a source of amino acids that act to improve matrix structure. Although this study describes an effect of collagen in the management of OA pain, factors such as variation in the degree of disease progression at the time of inclusion in the study, the inclusion of hips and knees as joints to be analyzed, the use of a not widely used outcome measurement scale, and a significant dropout rate represent caveats in the interpretation of research results (15).
Another study (16) administered daily 10 g of hydrolyzed collagen to over 100 patients from 1 to 6 months. Participants receiving the collagen had significantly higher plasma levels of hydroxyproline and a major component of collagen than those in the placebo group. Although these studies were open-label trials, which means that there is a limited level of scientific evidence, we can see the high degree of safety of use with a dose of 10 g/day of pharmaceutical grade hydrolyzed collagen.
Luo et al. (17) conducted a study in patients with knee osteoarthritis who received collagen, along with glucosamine and chondroitin sulfate in one group, with a control group who received placebo. At 12 weeks, the administration improved the experimental group regarding not only pain but also quality of life.
For Campbell et al. (18) intra-articular PRP is a viable treatment for knee OA and has the potential to lead to symptomatic relief for up to 12 months. There appears to be an increased risk of local adverse reactions after multiple PRP injections. Intra-articular PRP offers better symptomatic relief to patients with early knee degenerative changes. In the short-term follow-up (≤ 1 year), intra-articular PRP injection is more effective in terms of pain relief and function improvement to treat patients with knee OA than HA and placebo (17), and there is no difference in the risk of an adverse event between PRP and HA or placebo (20). Di Martino et al. (21) enrolled 167 patients with knee OA (Kellgren-Lawrence grade 0-3) randomized to undergo 3 blinded weekly intra-articular injections of either PRP or HA. Patients were prospectively assessed before treatment and for a median follow-up of 64 months. Both treatments effectively improved the knee functional status and symptoms over time up to 24 months. The PRP group still presents significantly higher values compared to baseline and HA though not statistically significant compared to baseline. A comparative analysis showed no significant intergroup difference in any of the clinical scores at any follow-up point. The median duration of patient subjective perception of symptomatic relief was 9 months for HA and 12 months for PRP. The latter did not provide an overall superior clinical improvement compared to HA in terms of symptomatic-functional improvement at different follow-up points or effect duration. Filardo et al. (22) included 192 patients with unilateral symptomatic knee with chronic pain or swelling with a Kellgren-Lawrence score of 0-3 on the X-rays. Patients underwent 3 weekly intra-articular injections of either PRP or HA. Both treatments proved effective improving the knee functional status and reducing symptoms with IKDC scores being obtained in both the PRP group and in the HA group. The comparative analysis of the 2 treatments showed no significant intergroup difference at any follow-up evaluation in any of the clinical scores used.
Hydroidan Pro is composed by 10 g of hydrolyzed collagen with 100 mg fucoidan. Fucoidan is a generic term for a class of molecules that are a class of polysaccharides composed of a main chain of fucose, fucopyranose, and natural sulfate, which are found in brown algae (echinoderms) and account for over 40 % of the dry weight of cell walls of algae. They have a wide spectrum of activity in biological systems. Its main function is to form a gel network to protect the floating structures of algae from drying out as they are exposed to air while their root and much of the stem are submerged in seawater (23). Fucoidan can be used in cosmetics, functional foods, dietary supplements, and in pet, livestock and aquaculture food supplements. To date, fucoidan has not been developed as a regulated therapeutic product yet. However, research on the use of fucoidan, specifically the one extracted from Fucus vesiculosus, has recently gained interest due to its biological activities and potential medical applications (24). Animal models of collagen-induced arthritis showed that orally administered fucoidan successfully inhibited pain (25). One of the physio-pathological components of great importance in the etiology of pain and inflammation in OA is the one associated with selective blocking of the migration and accumulation of neutrophils to the joint and the release of inflammatory mediators. This is related to its action on adhesion molecules found in platelets (P-selectins), and leukocytes (L-selectins), which theoretically prevents the passage of inflammatory cells to tissue spaces, attenuating inflammation. Carvalho et al. (23) demonstrated the effect of fucoidan to inhibit neutrophil infiltration and reduce the levels of pro-inflammatory cytokines and the symptoms of OA were inhibited by 52 % during 12 weeks of oral administration. There was no reduction of TNF-ß as a marker of inflammation. However, an accompanying study with healthy volunteers showed a reduction of IL-6, a marker of chronic inflammation. In an injection-induced arthritis model of zymosanÒ, a carbohydrate used to induce sterile inflammation experimentally, in knees of rats, the administration of a fucoidan preparation (F. vesiculosus) administered at intraperitoneal doses of 15 mg/kg, 30 mg/kg, and 50 mg/kg, 1 hour after the induction of joint inflammation reduced cell migration significantly. The dose of 30 mg/kg also improved the loss of glycose-minoglycans caused by zymosan®.
A small clinical trial (24) explored the use of a preparation of fucoidan (85 % w/w), Macrocystis pyrifera (10 % w/w) and Laminaria japonica (5 % w/w) in 12 subjects with OA randomized to take either 100 mg or 1000 mg capsules (75 mg or 750 mg fucoidan) orally for 12 weeks. There was a dose-response improvement in the participants. The lower dose reduced the average score by 18 %, while the higher dose improved the score by 52 %. They found also a reduction of leukotriene B4 and prostaglandin E2. The reduction of mediators of inflammation was associated with a reduction of joint pain. Predominant cells related to the inflammatory process induced in the ankle were granulocytes, not lymphocytes. The injection of fucoidan (10 mg/kg) reduced interactions between leukocytes and endothelial cells, and the number of cells decreased by nearly 60 % (26).
Intra-articular collagen injection with ChondroGrid (CG) a hydrolyzed (< 3 kDa) bovine collagen injectable formulation was injected in 70 patients affected by Kellgren Lawrence grade 1 to 4 knee OA and BMI < 30. These patients were given 3 CG injections and followed up for 6 months after the last administration. At the last follow-up, they showed a 50 % reduction of their median Lequesne score, a 50 % reduction of their VAS score at rest and in motion, and a ≥ 50 % reduction in all other scores under consideration (27). De Luca et al. (28) studied 20 patients with Kellgren Lawrence grade 1 to 4 knee OA who received three 4 mg/2mL injections of bovine hydrolyzed < 3 kDa type I collagen (ChondroGrid), 2 weeks apart. These patients were retrospectively assessed to compare scores collected before and 15, 45, and 225 days after the first injection. ChondroGrid induced type-II and inhibited type-I collagen deposition. Patients showed a 44 % Lequesne score and a 55 % VAS at moving score reduction.
Comblain et al. (29) demonstrated that a mixture of curcuminoids extract, hydrolyzed collagen, and green tea extract (COT) inhibited inflammatory and catabolic mediator's synthesis by osteoarthritic human chondrocytes. The compounds were more efficient inhibiting the interleukin-1β stimulated matrix MMP-3 expression than curcuminoids extract alone. In interleukin-1β-stimulated human chondrocytes, nitric oxide, interleukin-6 and matrix MMP-3 productions reduced significantly with curcuminoids extract alone or together with hydrolyzed collagen and green tea extract (14). The COT mixture has beneficial effect on OA physiopathology by regulating the synthesis of key catabolic, inflammatory and angiogenesis factors.
Study limitations: the groups couldn't be completely blind because in one of them blood was drawn while in the other 2 it wasn't. It would be necessary to continue the study for a longer time to know if there are imaging changes. Ultrasonography or MRI could be useful for evaluation purpose. However, it complicates the control of these patients. On the other hand, they are not exclusive treatments, so the combination of intra-articular injections of HA or PRP along with oral treatment of hydrolyzed collagen should be tried.
Prolonged treatments with oral collagen, in combination or not with other therapies, can be useful in patients with OA to slow down the degenerative process with the occurrence of few adverse events. Few clinical scientific studies have been published to this date, so we recommend going deeper into their results to better understand their mechanism of action.
Hydrolyzed collagen along with fucoidan, taken orally daily for 12 weeks, seems to have better results in the WOMAC and VAS scales compared with intra-articular therapies such as hyaluronic acid or platelet-rich plasma. Combined oral and intra-articular therapies should be tried to determine the efficacy profile.