Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista de Osteoporosis y Metabolismo Mineral
versión On-line ISSN 2173-2345versión impresa ISSN 1889-836X
Rev Osteoporos Metab Miner vol.15 no.4 Madrid oct./dic. 2023 Epub 19-Feb-2024
https://dx.doi.org/10.20960/revosteoporosmetabminer.00024
REVIEW
Function of sex hormones in bone homeostasis and their role in the development of male osteoporosis: a narrative review
1Departamento de Ciencias de la Acupuntura. Universidad Estatal del Valle de Ecatepec. México
2Unidad de Ciencias de la Salud. Universidad ETAC Campus Coacalco. México
3Laboratorio de Genómica del Metabolismo Óseo. Instituto Nacional de Medicina Genómica (INMEGEN). México
Bone is a dynamic tissue that undergoes constant adaptation throughout the life of vertebrates to achieve size, shape, preserve the structural integrity of the skeleton, and regulate mineral homeostasis. Bone growth during childhood is crucial to achieve height and resistance to fractures later in life. Sex hormones play a key role in bone remodeling in men and women alike, and changes to hormonal profiles can trigger bone metabolism-related diseases. In women, estrogen deficiency during menopause is one of the leading causes of osteoporosis, while in men, androgens can have an impact on bone health by binding directly to androgen receptors or indirectly to estrogen receptors.
This review explores the role and effects of sex hormones on bone metabolism, the signaling pathways involved, and the effects that can trigger diseases such as osteoporosis.
Keywords: Male osteoporosis; Androgens; Estrogens; Testosterone
INTRODUCTION
Osteoporosis (OP) is one of the most common metabolic diseases across the world. OP is characterized by the loss of bone mass and the deterioration of bone microarchitecture, predisposing patients to sustaining fragility fractures (1). OP is considered a subclinical condition until it becomes complicated with a fracture, which poses a medical, personal, and high socio-economic burden, and the use of several resources required for the management of affected individuals (2,3). OP is often considered a condition that affects postmenopausal women. However, in recent years, it has been reported that one-third of all hip fractures sustained occur in men, and the incidence of vertebral fractures can exceed more than half of those reported in women (4,5). Currently, it is estimated that 75 million people in Europe, the United States, and Japan are affected by OP, leading to up to 8.9 million fragility bone fractures. In Mexico, according to data (2010) from the population and housing census bureau, the overall population was 112 million people, 17% of which corresponded to the adult population older than 50 years. Within this population, 17% of Mexican women and 9% of Mexican men showed OP in their lumbar spines, while 16% of women and 6% of men showed OP in their hips, respectively (5,6). Currently, according to data from the 2020 population and housing census bureau, the Mexican population (126 million people) faces an epidemiological transition with an increased life expectancy (overall, 17.46% of the population are already older than 50 years). It is estimated that 10 million individuals are living with OP, meaning that 1 out of every 3 women and 1 out of every 5 men will end up developing OP (7,8). According to these statistics, male OP is considered a growing reason for concern regarding public health, thus prompting the development of clinical practice guidelines for the management of this disease that now address the management and treatment of OP in male patients as well. However, despite the drafting of these guidelines, male OP is still considered an underdiagnosed and undertreated disease (9). Although the clinical signs between men and women are similar, some characteristics are specific to male OP. For example, in most cases, the type of OP is “secondary,” meaning it originates as a direct consequence of other diseases, use of drugs, or lifestyle changes. The densitometry criteria for the diagnosis of OP are not well validated, and studies on the effect of various treatments to prevent fractures in men are lacking. There are no health records on trauma, or on the origin fractures either; additionally, men are less prone to falling. Also, men's life expectancy is shorter, meaning that the therapeutic actions used in men are different from those used in women (10). Therefore, the objective of this study is to conduct a narrative review on the role of sex hormones, their impact on bone mineral density, and their role in the development of male OP.
BONE GROWTH
Bone is a highly specialized type of connective tissue whose main function is to provide mechanical support for muscle activity and physical protection of internal tissues and organs. Also, it plays a key role maintaining homeostasis and serves as a mineral reservoir at systemic level (11). During growth, significant differences can be seen between men and women in the early stages of life. Bone growth is impacted by various factors such as sex hormones, the level of physical activity, and body size. Adolescence is the stage that possibly has the greatest impact on the formation and development of the skeletal system in both men and women. During puberty, boys enter this stage later than girls, and it lasts longer, which could lead to differences in bone growth between the 2 genders. An example of this is that men tend to have longer legs than women because epiphyseal fusion occurs later in men due to a longer period of bone maturation (12). On the other hand, sex hormones also have an impact on growth, bone homeostasis, and the completion of bone maturation. In men, testosterone plays a key role in developing larger skeletons, while estrogen has been associated with less bone resorption, thereby preserving bone mass. However, it has been reported that testosterone also improves bone formation and may be associated with less bone tissue resorption, which could be due to the conversion of testosterone into estrogen, suggesting that while estrogens may be responsible for preserving bone mass, testosterone may be responsible for increasing it. These functions of sex hormones in male development bring about several advantages as they reach adulthood. They help protect bones from fragility fractures compared to women, allowing them to achieve a much higher peak bone mass, larger bone size, and greater bone strength (13).
STROGENS INVOLVED IN BONE GROWTH
Estrogens are a family of steroid hormones including estradiol, estriol, and estetrol. Estradiol (E2) is the most common and active estrogen there is, and is basically produced by the ovaries. However, adipose tissue, testes, the suprarenal cortex, and the liver also contribute to its production. Estrogens have been associated with maintaining bone mass, and clinical observations have established that estrogen deficiency in bone mass is also a cause of OP in men, suggesting their universal role in bone metabolism (14). The effects of estrogens on bone mass are mainly attributed to their activity inhibiting osteoclast-induced bone resorption. Various in vitro studies using osteoblast cell lines and stromal cells show that estrogens reduce the production of osteoclastogenic cytokines and increase the expression of factors that inhibit osteoclastogenesis (15,16). Still, the effect estrogens may have on bone formation remains unclear, as stromal cells and osteoblasts express estrogen receptor alpha (ERα) and beta (ERβ), which can affect differentiation and bone formation. Osteoblasts are derived from mesenchymal stem cells (MSCs), which can also produce adipocytes, suggesting a regulatory mechanism that determines the lineage between osteoblasts and adipocytes, which could be a critical component in the regulatory pathway of osteoblastogenesis (17). Increased lipid concentration in the bone marrow has been associated with age-related bone loss, which involves the existence of an inverse relationship between adipogenesis and osteoblastogenesis (18). Additionally, it has been reported that ovariectomy-induced osteopenia is associated with an increased adipogenesis. Therefore, in various in vitro studies, it has been hypothesized that estrogens negatively regulate the expression of lipoprotein lipase (LPL), a typical marker of adipocyte differentiation. Therefore, estrogens may be regulating bone formation by inactivating the bone marrow stromal cells of mesenchymal origin to induce lineage switching toward osteoblasts (19). On the other hand, it is believed that the primary goal of estrogens is to inhibit osteoclast-induced bone resorption. Osteoclasts are multinucleated giant cells whose primary function is to degrade the bone mineral matrix during the bone remodeling resorption phase (20). The recruitment of osteoclast precursors, differentiation, and resorption activity are controlled by local factors such as vitamin D, prostaglandins, TGF-β, IL-1, IL-6, and TNF-α, which stimulate osteoclast differentiation and activity through direct or indirect mechanisms. Calcitonin, however, inhibits their activity. The fate of osteoclasts after bone resorption is still unknown. Factors like calcitonin inactivate osteoclasts without inducing cell death, whereas bisphosphonates and vitamin K2 induce osteoclast cell death. The effect estrogens have on osteoclasts is believed to be indirectly regulated through non-osteoclastic cells. Lower estrogen levels during menopause or due to ovariectomy are associated with elevated levels of IL-1, IL-6, and TNF-α and lower levels of TGF-β due to peripheral blood monocytes, bone marrow stromal cells, and osteoblasts (21). Other factors are also involved in osteoclast differentiation and activation, being the receptor activator of nuclear factor kappa B (RANK) one of the key signaling pathways. RANK, which is expressed in osteoclasts, becomes activated when it binds to the receptor activator of nuclear factor kappa B ligand (RANKL). In this mechanism, the osteoprotegerin (OPG) protein also serves as a decoy for RANKL, suppressing osteoclast differentiation activation. Estrogens can regulate RANKL and promote the expression of OPG, thus reducing bone resorption by changing the expression of human osteoblast cellular proteins, including some members of the Wnt/β-catenin signaling pathway, which negatively regulate osteoclastogenesis and mediate anabolic effects on the bone (22,23). Additionally, estrogens inhibit osteoclast differentiation and promote osteoclast apoptosis by increasing the production of TGF-β. In the absence of estrogens, RANKL expression is induced, thus triggering osteoclastogenesis (24) (Fig. 1A).
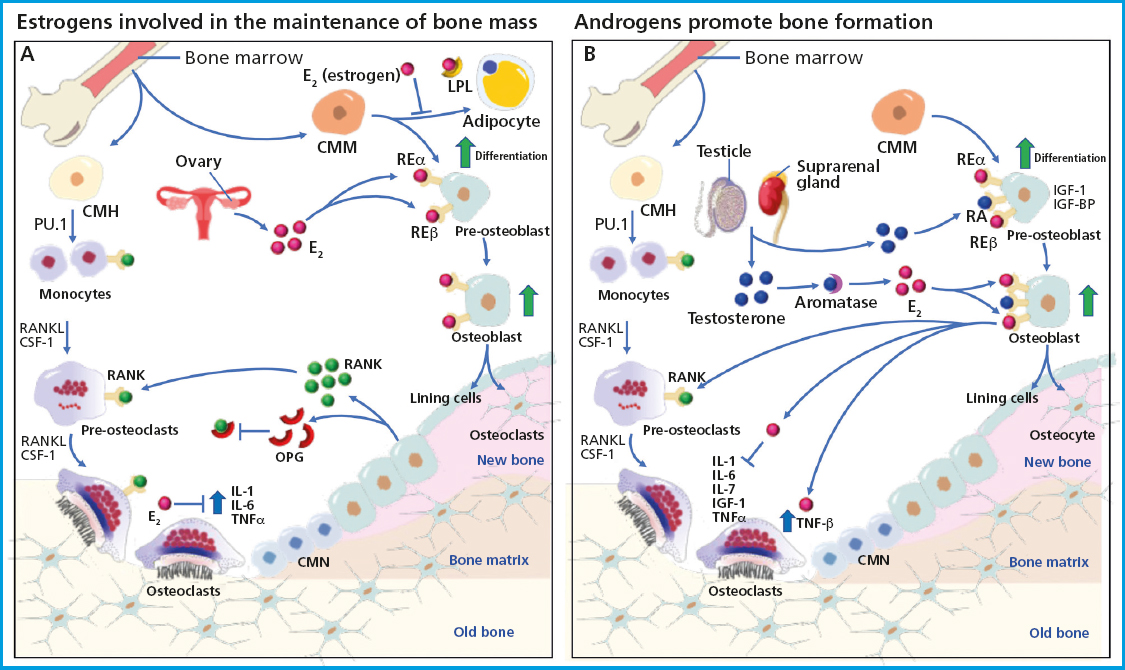
Figure 1. Schematic representation of basic multicellular units (BMUs), their association with bone remodeling, and the role of sex hormones in bone tissue maintenance and growth. The activation of bone remodeling starts with the differentiation of hematopoietic stem cells into mature osteoclasts capable of resorbing bone tissue, where cytokines CSF-1 and RANKL are required at all stages of differentiation. Once bone resorption is completed, there is an attraction of mononuclear cells towards bone remodeling units to recruit osteoblast precursors that will later differentiate into mature osteoblasts and osteocytes, which will become trapped inside the mineralized matrix and function as chemotaxis and mechanosensors. Osteoblasts are bone-forming cells from mesenchymal stem cells and require the activity of various factors for their differentiation, such as sclerostin, the transcription factor RUNX2, and the activation of IGF-1 and IGF-BP proteins. A. It shows the production of estrogens by the ovaries, capable of inhibiting the activity of cytokines IL-1, IL-6, and TNF-α, which are necessary during osteoclastogenesis. In addition, estrogens can bind to the ERα and ERβ present in osteoblast precursors to promote their differentiation and in mature osteoblasts to secrete osteoclastogenesis-inhibiting cytokines such as osteoprotegerin (OPG), which functions as a decoy receptor for RANKL, preventing its binding to osteoclasts and maintaining bone mass. B. It depicts the activity of the testosterone secreted by the testes and the suprarenal gland, which binds to androgen receptors present in osteoblasts and promotes differentiation into mature osteoblasts. Testosterone also acts as an inhibitor of IL-6, which is necessary for osteoclastogenesis, and is turned into estrogens through aromatase, thus promoting osteoblastic differentiation. Additionally, testosterone regulates the expression of cytokines necessary for osteoclastogenesis, thus contributing to the formation of bone tissue.
THE ROLE OF ANDROGENS IN BONE GROWTH
The term “androgen” refers to testosterone and its cholesterol-derived precursors. Testosterone is a predominant androgen in men, secreted in 95% by the testes and 5% by the suprarenal glands through the conversion of dehydroepiandrosterone (25). Testosterone binds to albumin and sex hormone-binding globulin to allow for its local conversion to 5α-dihydrotestosterone (DHT) through peripheral tissues, which have a high affinity due to the abundance of androgen receptors (AR) they have. Testosterone exerts strong anabolic and androgenic effects that impact both men and women, significantly influencing bone growth and maintenance. A study demonstrated that the administration of testosterone in murine models led to a wider epiphyseal growth plate, and these effects were independent of growth hormone and insulin-like growth factor-1 (IGF-1). Similarly, the role of testosterone in bone growth was seen (26). Testosterone has also been shown to play a crucial role in maintaining bone mineral density (BMD) in older men (27). However, serum testosterone levels in older men decrease by 1% per year, which may lead to the clinical symptoms of late-onset hypogonadism (LOH), which is characterized by depression, irritability, sexual dysfunction, decreased lean body mass, and decreased BMD, which may be associated with aging. Therefore, testosterone replacement therapy has been proposed to improve the quality of life of older men with LOH (28). As mentioned earlier, estrogens are necessary for BMD maintenance, and in women, estrogen levels decrease significantly during menopause. In contrast, testosterone levels in men decrease slowly with age, allowing for the stable maintenance of BMD over a longer period of time, which is why OP is more common in postmenopausal women than older men (29). In bone metabolism, testosterone plays a crucial role as it is converted into highly active DHT through 5α-reductase in the cytoplasm of target cells, allowing it to bind to androgen receptors (AR) and inducing androgenic activity. Additionally, testosterone can also be converted into E2 due to aromatase activity, which allows it to bind to estrogen receptor subtypes (ERα and ERβ), which are associated with bone metabolism. ARs are present in chondrocytes and osteoblasts, with their expression levels varying depending on each individual's age and bone sites. The binding of testosterone to ARs in osteoblasts promotes bone formation through the indirect activation of cytokines and growth factors. Osteoblasts synthesize various cytokines that promote bone resorption, such as IL-6 and TNF (30). Androgens also positively regulate the TGF-β and IGF growth factors that stimulate bone formation (31). It has been reported that testosterone deficiency promotes the expression of RANKL in osteoblasts, subsequently activating osteoclast differentiation and increasing bone resorption, resulting in reduced BMD. Chondrocyte and osteoblast differentiation and proliferation are induced by the binding of IGF-1 to insulin-like growth factor-binding protein (IGF-BP), which along with chondrocyte apoptosis suppression promotes bone formation. Therefore, testosterone positively regulates the expression of IGF-1 and IGF-BP in osteoblasts (32). Testosterone can also regulate osteoclastogenesis by suppressing the activity of interleukin (IL) 6, which is responsible for osteoclast activation and bone resorption. Therefore, lower levels of testosterone negatively affect BMD. Interestingly, higher levels of AR expression have been reported in osteocytes, the most abundant cells inside the bone, which have been shown to produce various mediators that can influence osteoclastogenesis, such as nitric oxide, TGF-β, prostaglandins, or RANKL. Estrogen and androgen deficiencies lead to a higher prevalence of osteocyte apoptosis (33), which can indirectly stimulate osteoclastogenesis by inducing stromal/osteoblastic cells to secrete RANKL. Additionally, osteocytes secrete OPG, which competes with RANK for its receptor on osteoclasts. Osteocytes, like osteoblasts, regulate the secretion of OPG through the Wnt/β-catenin signaling pathway. Mice lacking β-catenin in osteocytes have been reported to be osteoporotic due to an increased number of osteoclasts, a mechanism similar to that reported in humans. Osteocytes control the bone remodeling process by directly and indirectly regulating osteoclast and osteoblast differentiation and function. Any disruption in this process leads to osteoporosis. In this regard, estrogen receptor subtypes ERα and ERβ play a key role in maintaining BMD in men, as estrogens have a greater effect than androgens inhibiting bone resorption. The loss of ERα function and aromatase deficiency in men induce the development of a phenotype with an extremely low BMD, thus leading to estrogen replacement therapy as an option to improve the levels of BMD in adult male patients (35). E2 is often responsible for regulating osteoclast apoptosis and the function by increasing the expression of tumor growth factor β (TGF- β). Also, the expression of IL-1, IL-6, IL-7, IGF-1, nuclear factor κB (NF-κB), RANK, and tumor necrosis factor α (TNFα) increases, thus reducing osteoblast proliferation and activity. These genes are known targets of the anti-resorptive effect estrogens have on the bone (36) (Fig. 1B).
RELATIONSHIP BETWEEN SEX HORMONES AND BONE FRACTURES
Falls and fractures are a common thing in older men while performing activities of daily living. The search for tools to help prevent fragility fractures has become a major global objective. The occurrence of age-related fractures is primarily due to reduced physical function, including loss of lean body mass, muscle weakness, bone fragility, sarcopenia, and decreased BMD. Recent studies have identified the relationship that exists between testosterone and the risk of fractures. Also, it has been reported that older male patients with osteoporotic fractures have very low testosterone levels compared to control groups of the same age and ethnicity (37,38). Some studies support the hypothesis that testosterone deficiency is associated with an increased incidence of falls, while others reject this hypothesis (39). The most predominant bone fractures associated with a reduced BMD following low testosterone levels can also be due to the relationship among testosterone, muscle strength, and physical performance in men, which could lead to the development of sarcopenia and a higher risk of falling. Currently, it has been established that the relationship between testosterone deficiency and low BMD is much stronger in young adult men with moderate-to-severe hypogonadism (40). However, few studies have been published on the epidemiology of male OP, which may be due to the small sample sizes and potential biases of these studies. Case-control trials comparing the prevalence of hypogonadism between subjects with OP and control groups have shown that OP-induced fractures are more common in patients with hypogonadism compared to patients without this condition (41). Other studies have documented a significant increase in the risk of fragility fractures among patients with low levels of testosterone and E2. These low levels of sex hormones are associated with muscle atrophy and a reduced total lean body mass. Therefore, it is logical to assume that a loss of muscle function can impair the protective mechanism against falls, thus leading to an increased incidence of fractures in male patients. Currently, it has become widely accepted that bone metabolism disorders in patients with low estradiol levels can increase the risk of fractures, which could be due to a deficit in the transformation of testosterone to estradiol due to aromatase enzyme dysfunction. Some studies have even reported the development of severe male OP due to mutations in the estrogen receptor of the aromatase enzyme (42).
SIGNALING PATHWAYS IN SEX HORMONE-ACTIVATED BONE METABOLISM
E2 and other steroid hormones are capable of inducing the activation of different signaling pathways by binding to their receptor through 3 mechanisms: a) classical signaling, where E2 binds to ERα and ERβ in the cytoplasmic compartment, and then this complex moves to the nucleus where it forms homo- or heterodimers that directly bind to a specific DNA sequence called estrogen response elements (EREs); b) ERE-independent signaling, where the E2/ER complex moves to the nucleus and interacts with transcription factors to sequester them and change their interaction with DNA, leading to changes in gene expression; and c) non-genotropic signaling (not involving changes to gene expression), in which E2 sends signals through a G protein-coupled receptor (GPCR) on the plasma membrane. ERs are highly expressed in bone, and their effects have been attributed to receptor-mediated activity. These effects were demonstrated in a study where a group of ovariectomized female mice (OVX) with ERα-/- and a group of orchidectomized male mice (ORX) with ERα-/- did not respond to exogenous estrogen treatment. ERα-/- mice showed about a 10-fold increase in E2 levels and 5 times higher levels of testosterone, as well as impaired IGF-1 levels, leading to an increased osteoclast activity and, therefore, the development of an osteoporotic phenotype (43). Both the nucleus and the cell membrane have ERα receptors, which activate transcription-independent signaling pathways that are activated by non-genomic mechanisms of ERα, where estrogen exerts antioxidant effects independently. The biological effect of osteogenesis is associated with highly specific cellular signaling pathways, including the phosphatidylinositol-3-kinase (PI3K) signaling pathway and protein kinase B (Akt), both of which play critical roles in osteoblasts and bone formation by regulating fundamental cellular processes. The interaction between E2 and ERα activates the PI3K-Akt signaling pathway, where the PI3K protein is a heterodimeric enzyme made up of a catalytic subunit (P110) and a regulatory subunit (p85), which are necessary for a wide range of cellular activities, including metabolism and aging. On the other hand, Akt is a phosphoinositide-dependent serine/threonine protein kinase. The subsequent interaction of PI3K and Akt are crucial regulators of bone resorption and bone formation by osteoclasts, promoting their differentiation and survival for the maintenance and turnover of bone mass. The deficiency of Akt in osteoblasts induces an apoptosis-susceptible phenotype and suppresses cellular function and differentiation, which is why the PI3K-Akt signaling pathway plays a key role in the bone formation process on the cell membrane (44). On the other hand, the E2/RE interaction promotes the activation of the mitogen-activated protein kinase (MAPK) signaling pathway, which consists of a set of serine/threonine kinases that regulate a wide range of stimuli. ERK has 2 different isoforms, ERK1 (MAPK3) and ERK2 (MAPK1), both of which are expressed in osteoblasts. ERK is activated by MAP2Ks-MEK1 (MAP2K1) and MEK2 (MAP2K2). Mice with germline deletion of Erk1 and conditional deletion of Erk2 in limb mesenchyme (Erk-1-/-Erk2Prx1 mice), including osteoblasts, exhibit a substantial reduction in bone mineralization, demonstrating the importance of ERK in osteoblast mineralization. Similarly, mice expressing the dominant MEK1 mutation in osteoblasts exhibit low bone mass and hypomineralization of the clavicle and cranial vault. In particular, these mice also display clavicular and cranial hypomineralization, which are reminiscent of mice and humans haploinsufficient for Runx2, the master regulator of osteoblast differentiation (45) (Fig. 2). Another signaling pathway stimulated by the binding of testosterone to the androgen receptor is the renin-angiotensin system (RAS). It has been reported that RAS is a complex system that acts as a mediator between bone formation and resorption through various mechanisms. The role of RAS begins with the conversion of angiotensinogen into angiotensin I (AngI), which is activated by renin, a highly selective protease secreted by the juxtaglomerular cells of the kidney. Afterwards, AngI is converted into angiotensin II (AngII) through the angiotensin-converting enzyme (ACE). The relationship between the renin-angiotensin system and bone metabolism is primarily based on the regulation of AngII in bone. It has been reported that AngII is associated with a significant increase in TRAP-positive osteoclasts and positive regulation of RANKL expression through the extracellular kinase of osteoblasts (46) (Fig. 3). However, these effects are repressed with treatment targeted at ACE inhibition or angiotensin type 1 receptor blockers (ARBs), making the RAS signaling pathway emerge as a strategy in the treatment of bone metabolism disorders such as osteoporosis (47). Currently, the management of OP in men is no different from that indicated in women, and few studies have been conducted on the efficacy of drugs in men. The non-pharmacological treatment of OP is essentially based on lifestyle and does not change between men and women (48). However, the Endocrine Society has formulated specific clinical practice guidelines on the management of male OP, such as bisphosphonates, which are targeted at patients with recent hip fractures, and teriparatide for patients with GI problems and a high risk of fracture. On the other hand, the North American Menopause Society (NAMS) has suggested changes in dietary habits, lifestyle, and initiating pharmacological treatment with bisphosphonates for the management of postmenopausal women—as first-line options—and raloxifene in younger postmenopausal women, to prevent bone loss and reduce the risk of vertebral fractures (49,50).
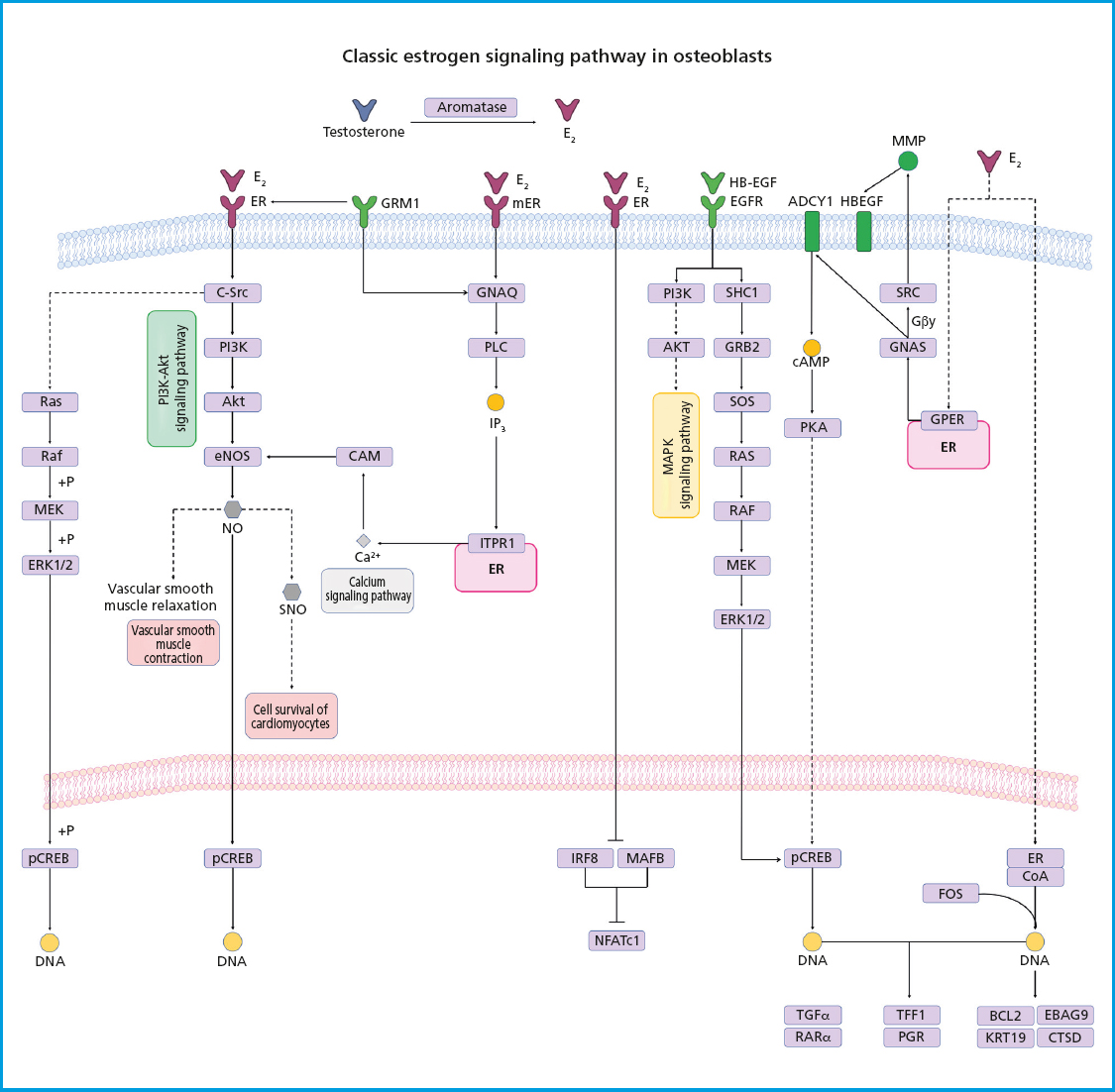
Figure 2. Estrogen receptor signaling. Estrogens diffuse through the plasma membrane binding to ERα or ERβ with nuclear dimerization and translocation. ERs bind to specific sequences, recruit coactivators, and transcribe their target genes. Estrogen-bound ERs can also interact with transcription factors such as AP1, SP1, and NF-kB, which also play a significant role in regulating osteoclastogenesis. The activation of tyrosine kinase receptors (EGFR) and G protein-coupled receptors (GPCRs) leads to the activation of MAPK, PKA, and PI3K-Akt signaling pathways.
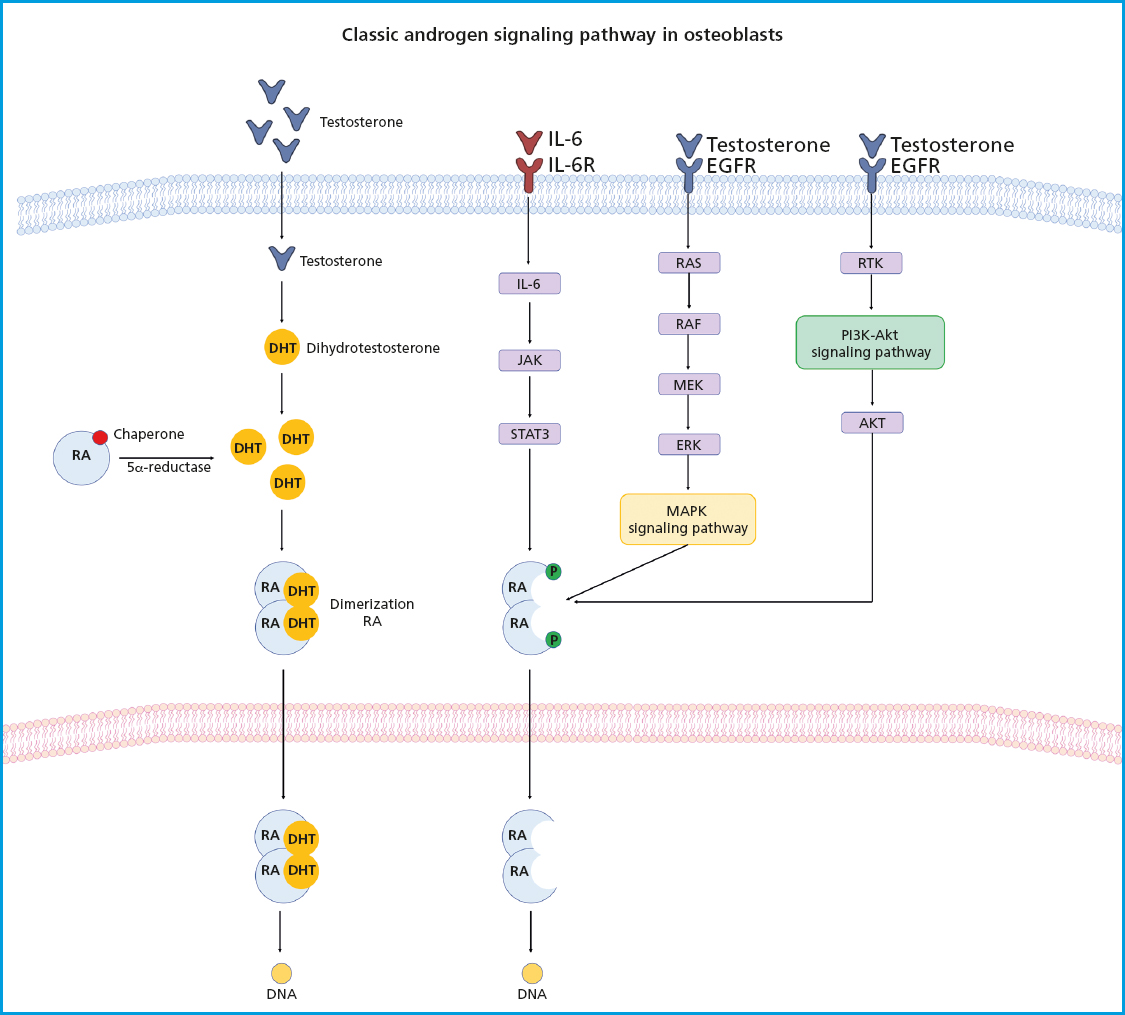
Figure 3. Androgen receptor signaling. The androgen receptor (AR) is kept inactive in the cytoplasm by chaperone proteins. The binding of androgens to the AR lead to the dissociation of the chaperone complex, causing a conformational change in the AR followed by its dimerization. The AR dimer translocates to the nucleus and binds to promoters/enhancers of target genes, thus facilitating transcription through interactions with coregulators. ARs can also affect cell signaling without directly binding to gene promoters. In the absence of androgens, various growth factors and cytokines can also activate the AR by regulating multiple signaling pathways, including the PI3K-Akt and MAPK/ERK-mediated RAS pathways involved in bone formation.
On the other hand, the effect of androgens on surrogate markers such as TBS or micro-CT has been poorly studied. In a study conducted by Cauley et al. in 2021, the BMD of 211 older men who received moderately low testosterone treatment, without any other reason than age, was analyzed. It was reported that testosterone treatment for 1 year, compared to a control group, significantly increased volumetric trabecular BMD levels. The results were analyzed through quantitative computed tomography (QCT) of the hip and spine, showing an increased estimated bone strength. However, the authors mention that QCT scans are expensive, involve high levels of radiation, and are unlikely to be added to routine clinical practice. Therefore, they propose the use of trabecular bone score (TBS) as an indirect measure of vertebral spine bone microarchitecture, which can be obtained from texture analysis of routine lumbar spine DXA scans and, along with the FRAX® prediction tool, can enhance fracture prediction accuracy and improve an individualized clinical management of OP (51). On the other hand, a study conducted by Movérare et al. in 2003 sought to compare the effect of ER activation on bone in vivo with the effect of AR activation in 9-month-old orchiectomized wild-type mice with ER inactivated by the androgen 5α-dihydrotestosterone. QCT analysis of BMD demonstrated that the bone preservation effect of ER activation and AR activation was of the same degree. However, a more detailed analysis of trabecular bone microarchitecture, using high-resolution micro-CT, showed that ER activation, as opposed to AR activation, preserved trabecular thickness, while AR activation only preserved the number of trabeculae (52). Therefore, these tools can be used to create computer simulations of bone remodeling and dynamically assess a response to testosterone therapy in routine clinical practice.
CONCLUSIONS
The bone is a tissue that undergoes constant renewal through the process of bone resorption and formation. However, disruptions in this process can lead to the development of diseases like OP. While many studies have recognized the role of estrogen and its interaction with specific receptors as regulators of bone metabolism, androgens have been less examined. Evidence suggests that androgens like testosterone play a key role in maintaining BMD and bone health in men. Additionally, it has been identified that many molecular mechanisms of testosterone operate on the signaling pathways involved in bone metabolism, including the PI3K-Akt, MAPK, and RAS pathways, which have been previously described for the role they play maintaining bone mass. Therefore, the role of testosterone could be explored as a treatment option to improve BMD in older men.
Acknowledgments:
the authors would like to thank Universidad Estatal del Valle de Ecatepec for the help provided while preparing this review.
REFERENCES
1. Porter JL, Varacallo M. OP. 2022 Sep 4. In:StatPearls (Internet). Treasure Island (FL):StatPearls Publishing;2023. [ Links ]
2. LeBoff MS, Greenspan SL, Insogna KL, Lewiecki EM, Saag KG, Singer AJ, et al. The clinician's guide to prevention and treatment of OP. Osteoporos Int 2022;33(10):2049-102. DOI:10.1007/s00198-021-05900-y [ Links ]
3. Clark P. OP in México “The challenge”. Salud pública de México 2009;51(1):S2-S3. DOI:10.1590/S0036-36342009000700002 [ Links ]
4. Peris-Bernal P. OP del varón. ¿Cómo diagnósticarla y tratarla? Rev Esp Reumatol 2001;28(3):135-42. [ Links ]
5. Clark P, Carlos F, Vázquez-Martínez JL. Epidemiology, costs and burden of OP in Mexico. Arch Osteoporos. 2010;5:9-17. DOI:10.1007/s11657-010-0042-8 [ Links ]
6. Narro-Robles J, Hernández-Bringas HH, Flores-Arenales R. El censo de población 2010:cuatro millones más de mexicanos de lo previsto. ¿El final de una política de Estado? Pap Poblac 2012;18(74):1-39. [ Links ]
7. Instituto Nacional de Estadística y Geografía e Informatica. Consultado en junio 2023. Disponible en:https://www.inegi.org.mx/app/ageeml/ [ Links ]
8. Clark P, Ramírez-Pérez E, Reyes-López A. Umbrales de evaluación para la detección de casos en riesgo de OP (OP) y fracturas por fragilidad con FRAX en población mexicana para el primer nivel de salud. Gaceta Médica de México 2016;152(S2):22-31. [ Links ]
9. Riancho JA, González-Macias J, Pérez-Castrillon JL. Guías de práctica clínica en la OP postmenopáusica, glucocorticoidea y del varón (actualización 2022). Rev Osteoporos Metab Miner 2022;14(1):13-33. DOI:10.4321/S1889-836X2022000100003 [ Links ]
10. Herrera A, Lobo-Escolar A, Mateo J, Gil J, Ibarz E, Gracia L. Male OP:A review. World J Orthop 2012;3(12):223-34. DOI:10.5312/wjo.v3.i12.223 [ Links ]
11. Stagi S, Cavalli L, Iurato C, Seminara S, Brandi ML, de Martino M. Bone metabolism in children and adolescents:main characteristics of the determinants of peak bone mass. Clin Cases Miner Bone Metab 2013;10(3):172-9. [ Links ]
12. Cossio-Bolanos M, Vidal-Espinoza R, Fuentes-Lopez J, Castelli Correia de Campos LF, Andruske CL, Urra-Albornoz C, et al. Reference values for bone density and bone mineral content from 5 to 80 years old in a province of Chile. Peer J 2022;10:13092. DOI:10.7717/peerj.13092 [ Links ]
13. Chen JF, Lin PW, Tsai YR, Yang YC, Kang HY. Androgens and Androgen Receptor Actions on Bone Health and Disease:From Androgen Deficiency to Androgen Therapy. Cells 2019;8(11):1318. DOI:10.3390/cells8111318 [ Links ]
14. Okazaki R, Inoue D, Shibata M, Saika M, Kido S, Ooka H, et al. Estrogen promotes early osteoblast differentiation and inhibits adipocyte differentiation in mouse bone marrow stromal cell lines that express estrogen receptor (ER) alpha or beta. Endocrinology 2002;143(6):2349-56. DOI:10.1210/endo.143.6.8854 [ Links ]
15. Marie JC, Bonnelye E. Effects of Estrogens on Osteoimmunology:A Role in Bone Metastasis. Front Immunol 2022;13:899104. DOI:10.3389/fimmu.2022.899104 [ Links ]
16. Kim HN, Ponte F, Nookaew I, Ucer Ozgurel S, Marques-Carvalho A, Iyer S, et al. Estrogens decrease osteoclast number by attenuating mitochondria oxidative phosphorylation and ATP production in early osteoclast precursors. Sci Rep 2020;10(1):11933. DOI:10.1038/s41598-020-68890-7 [ Links ]
17. Pierce JL, Begun DL, Westendorf JJ, McGee-Lawrence ME. Defining osteoblast and adipocyte lineages in the bone marrow. Bone 2019;118:2-7. DOI:10.1016/j.bone.2018.05.019 [ Links ]
18. Muruganandan S, Govindarajan R, Sinal CJ. Bone Marrow Adipose Tissue and Skeletal Health. Curr Osteoporos Rep 2018;16(4):434-42. DOI:10.1007/s11914-018-0451-y [ Links ]
19. Dang ZC, van Bezooijen RL, Karperien M, Papapoulos SE, Löwik CW. Exposure of KS483 cells to estrogen enhances osteogenesis and inhibits adipogenesis. J Bone Miner Res 2002;17(3):394-405. DOI:10.1359/jbmr.2002.17.3.394 [ Links ]
20. Bolamperti S, Villa I, Rubinacci A. Bone remodeling:an operational process ensuring survival and bone mechanical competence. Bone Res 2022;10(1):48. DOI:10.1038/s41413-022-00219-8 [ Links ]
21. Kameda T, Mano H, Yuasa T, Mori Y, Miyazawa K, Shiokawa M, et al. Estrogen inhibits bone resorption by directly inducing apoptosis of the bone-resorbing osteoclasts. J Exp Med. 1997;186(4):489-95. DOI:10.1084/jem.186.4.489 [ Links ]
22. Bord S, Ireland DC, Beavan SR, Compston JE. The effects of estrogen on osteoprotegerin, RANKL, and estrogen receptor expression in human osteoblasts. Bone 2003;32(2):136-41. DOI:10.1016/s∴-3282(02)00953-5 [ Links ]
23. Baron R, Kneissel M. WNT signaling in bone homeostasis and disease:from human mutations to treatments. Nat Med 2013;19(2):179-92. DOI:10.1038/nm.3074 [ Links ]
24. Cheng CH, Chen LR, Chen KH. OP Due to Hormone Imbalance:An Overview of the Effects of Estrogen Deficiency and Glucocorticoid Overuse on Bone Turnover. Int J Mol Sci 2022;23(3):1376. DOI:10.3390/ijms23031376 [ Links ]
25. Vanderschueren D, Laurent MR, Claessens F, Gielen E, Lagerquist MK, Vandenput L, et al. Sex steroid actions in male bone. Endocr Rev 2014;35(6):906-60. DOI:10.1210/er.2014-1024 [ Links ]
26. Phillip M, Maor G, Assa S, Silbergeld A, Segev Y. Testosterone stimulates growth of tibial epiphyseal growth plate and insulin-like growth factor-1 receptor abundance in hypophysectomized and castrated rats. Endocrine 2001;16(1):1-6. DOI:10.1385/ENDO:16:1:01 [ Links ]
27. Mohamad NV, Soelaiman IN, Chin KY. A concise review of testosterone and bone health. Clin Interv Aging 2016;11:1317-24. DOI:10.2147/CIA.S115472 [ Links ]
28. McBride JA, Carson CC, Coward RM. Diagnosis and management of testosterone deficiency. Asian J Androl 2015;17(2):177-86. DOI:10.4103/1008-682X.143317 [ Links ]
29. Shigehara K, Izumi K, Kadono Y, Mizokami A. Testosterone and Bone Health in Men:A Narrative Review. J Clin Med 2021;10(3):530. DOI:10.3390/jcm10030530. [ Links ]
30. O'Brien CA. Control of RANKL gene expression. Bone 2010;46(4):911-9. DOI:10.1016/j.bone.2009.08.050 [ Links ]
31. Thu HE, Mohamed IN, Hussain Z, Shuid AN. Dihydrotestosterone, a robust promoter of osteoblastic proliferation and differentiation:understanding of time-mannered and dose-dependent control of bone forming cells. Iran J Basic Med Sci 2017;20(8):894-904. DOI:10.22038/IJBMS.2017.9111 [ Links ]
32. Li X, Ominsky MS, Stolina M, Warmington KS, Geng Z, Niu QT, et al. Increased RANK ligand in bone marrow of orchiectomized rats and prevention of their bone loss by the RANK ligand inhibitor osteoprotegerin. Bone 2009;45(4):669-76. DOI:10.1016/j.bone.2009.06.011 [ Links ]
33. Manolagas SC, O'Brien CA, Almeida M. The role of estrogen and androgen receptors in bone health and disease. Nat Rev Endocrinol 2013;9(12):699-712. DOI:10.1038/nrendo.2013.179 [ Links ]
34. Kramer I, Halleux C, Keller H, Pegurri M, Gooi JH, Weber PB. al. Osteocyte Wnt/beta-catenin signaling is required for normal bone homeostasis. Mol Cell Biol 2010;30(12):3071-85. DOI:10.1128/MCB.01428-09 [ Links ]
35. Bellido T. Osteocytes and their role in bone remodeling. Actual Osteol 2013;9(1):56-64. [ Links ]
36. Almeida M, Laurent MR, Dubois V, Claessens F, O'Brien CA, Bouillon R, et al. Estrogens and Androgens in Skeletal Physiology and Pathophysiology. Physiol Rev 2017;97(1):135-87. DOI:10.1152/physrev.00033 [ Links ]
37. Kotwal N, Upreti V, Nachankar A, Hari Kumar KVS. A Prospective, Observational Study of OP in Men. Indian J Endocrinol Metab 2018;22(1):62-6. DOI:10.4103/ijem.IJEM_414_16 [ Links ]
38. Liu ZY, Yang Y, Wen CY, Rong LM. Serum Osteocalcin and Testosterone Concentrations in Adult Males with or without Primary OP:A Meta-Analysis. Biomed Res Int 2017;9892048. DOI:10.1155/2017/9892048 [ Links ]
39. Hsu B, Seibel MJ, Cumming RG, Blyth FM, Naganathan V, Bleicher K, et al. Progressive Temporal Change in Serum SHBG, But Not in Serum Testosterone or Estradiol, Is Associated with Bone Loss and Incident Fractures in Older Men:The Concord Health and Ageing in Men Project. J Bone Miner Res 2016;31(12):2115-22. DOI:10.1002/jbmr.2904 [ Links ]
40. Rochira V, Antonio L, Vanderschueren D. EAA clinical guideline on management of bone health in the andrological outpatient clinic. Andrology 2018;6(2):272-85. DOI:10.1111/andr.12470 [ Links ]
41. Vescini F, Chiodini I, Falchetti A, Palermo A, Salcuni AS, Bonadonna S, et al. Management of OP in Men:A Narrative Review. Int J Mol Sci 2021;22(24):13640. DOI:10.3390/ijms222413640 [ Links ]
42. Stumper NA, Wientgen H, Al-Hashimi L, Müller HW, Ohrndorf S, Gaber T, et al. Aromatase mutation in men as a rare cause of OP:a case report and review of the literature. Clin Exp Rheumatol 2023. DOI:10.55563/clinexprheumatol/gj7xal [ Links ]
43. Lindberg MK, Alatalo SL, Halleen JM, Mohan S, Gustafsson JA, Ohlsson C. Estrogen receptor specificity in the regulation of the skeleton in female mice. J Endocrinol 2001;171(2):229-36. DOI:10.1677/joe.0.1710229 [ Links ]
44. Zheng Z, He Y, Long L, Gan S, Chen S, Zhang M, et al. Involvement of PI3K/Akt signaling pathway in promoting osteogenesis on titanium implant surfaces modified with novel non-thermal atmospheric plasma. Front Bioeng Biotechnol 2022;10:975840. DOI:10.3389/fbioe.2022.975840 [ Links ]
45. Greenblatt MB, Shim JH, Glimcher LH. Mitogen-activated protein kinase pathways in osteoblasts. Annu Rev Cell Dev Biol 2013;29:63-79. DOI:10.1146/annurev-cellbio-101512-122347 [ Links ]
46. Nakagami H, Morishita R. Hormones and OP update. Effect of angiotensin II on bone metabolism. Clin Calcium 2009;19(7):997-1002. [ Links ]
47. Gebru Y, Diao TY, Pan H, Mukwaya E, Zhang Y. Potential of RAS inhibition to improve metabolic bone disorders. Biomed Res Int 2013;2013:932691. DOI:10.1155/2013/932691 [ Links ]
48. Rinonapoli G, Ruggiero C, Meccariello L, Bisaccia M, Ceccarini P, Caraffa A. Osteoporosis in Men:A Review of an Underestimated Bone Condition. Int J Mol Sci 2021;22(4):2105. DOI:10.3390/ijms22042105 [ Links ]
49. Watts NB, Adler RA, Bilezikian JP, Drake MT, Eastell R, Orwoll ES, et al. Endocrine Society. Osteoporosis in men:an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2012;97(6):1802-22. DOI:10.1210/jc.2011-3045 [ Links ]
50. Das S, Crockett JC. Osteoporosis - a current view of pharmacological prevention and treatment. Drug Des Devel Ther 2013;7:435-48. DOI:10.2147/DDDT.S31504 [ Links ]
51. CauleyJA, Ellenberg SS, Schwartz AV, Ensrud KE, Keaveny TM, Snyder PJ. Effect of testosterone treatment on the trabecular bone score in older men with low serum testosterone. Osteoporos Int 2021;32(11):2371-5. DOI:10.1007/s00198-021-06022-1 [ Links ]
52. Movérare S, Venken K, Eriksson AL, Andersson N, Skrtic S, Wergedal J, et al. Differential effects on bone of estrogen receptor alpha and androgen receptor activation in orchidectomized adult male mice. Proc Natl Acad Sci U S A 2003;100(23):13573-8. DOI:10.1073/pnas.2233084100 [ Links ]
Received: July 19, 2023; Accepted: November 03, 2023











 texto en
texto en 


