Introduction
Type 2 Diabetes (T2D) is one of the most prevalent diseases in low-income countries and is a direct cause of approximately 1.6 million deaths worldwide1. A family history of T2D (FH-T2D) and prolonged inadequate dietary habits are well established to be associated with future development of T2D2,3. Because T2D represents a major problem for public health4, there is a need to identify key patterns presented in young adults to prevent its development.
Anthropometry plays a fundamental role in evaluating an individual's nutritional status. Many reports have correlated certain anthropometric indices, based on body dimensions measurements, such as body-mass index (BMI), the corrected mid-arm muscle area index (MAMA), and tricipital skinfold (TSF) with increased energy reserves, which resulted from augmented adipose tissue at the expense of the fat/muscle mass ratio5. Deviations from ideal values can inform us about the development of metabolic disturbances that interferes with the use of nutrients from the diet6.
BMI is derived from the body's weight and height; however, a few studies have shown that BMI cannot discriminate between muscle and fat mass and as a consequence fails to identify where the body's fat (central versus peripheral) is located. Waist or hip circumference (WC and HC) and the waist-to-hip ratio (WHR) provide information on central fat content and leaves aside other regions of the body where fat can be stored, such as the hips and upper and lower extremities7. The subcutaneous tissue in peripheral locations along with visceral fat are the main storages of excess energy from the diet8. Therefore, it remains plausible that alternative indices that are not based on central obesity could aid the nutritional assessment when complement with traditional indices. Anthropometric indices based on the arm, such as MAMA, fat arm index (FAI), and TSF are nutritional indicators for the evaluation of fat and muscle reserves and could be integrated with routine anthropometric assessment to evaluate energy storages9,10.
The college lifestyle is marked with many major risk factors that are associated with the late development of T2D, such as but not limited to, a sedentary lifestyle, constant stress, alcohol consumption, lack of adequate sleep, and bad eating habits11,12. Regarding to this last point, different nutritional recommendations have emerged in order to maintain a correct diet and prevent the development of chronic diseases, such as T2D. These recommendations issued by different organizations (Bourges for the Mexican population, NOM-015 for the prevention and treatment of T2D, and the prevention goals for chronic disease established by the FAO/WHO) are a reference guide that allow us to identify and evaluate dietary characteristics, such as variety of foods, quantity and type of foods, as well as energetic distribution of macronutrients, that could play a role in the development of T2D13,14. Following these recommendations have shown a beneficial effect on weight control and biochemical parameters15,16. Even though these recommendations share many similarities, they are not identical; therefore, they should not be used individually but as an embodiment of rules for comparisons. On the other hand, it has been seen that those with a FH-T2D are more prone to obesity and b-cell dysfunction at younger ages17,18. They also present with a high percentage of poor eating habits19, which have changed over time and are currently characterized by increased consumption of industrialized products, fast food, saturated fats, simple carbohydrates, and decreased consumption of fiber, whole grains, as well as fruits and vegetables19. Therefore, under these situations, this could provide the right environment for the development of T2D in younger patients. For these reasons, it is important to recognize these factors early. Our main objective was to evaluate the dietary intake of Mexican medical students by comparing it with the most used recommendations for Mexico (Bourges for the Mexican population, NOM-015 for the prevention and treatment of T2D) and the prevention goals for chronic disease established by the FAO/WHO. Moreover, we also evaluate the effect FH-T2D, with and without, has on the association between dietary intake and assess if these groups are different anthropometrically.
Material and methods
Design and study population
Study population: This was a cross-sectional, descriptive, observational study carried out in Puebla, México using young university students from the medical school at the Benemérita Universidad Autónoma de Puebla. The recruitment and selection of the participants occurred during 3 different school period during 2017-2018: 2 cuatrimesters during the normal school year (January-April and August-November) and one summer session (May-June). Those students who wanted to participate gave written informed consent in agreement with the Declaration of Helsinki. This study was approved by the "research and postgraduate studies secretary" (ID: SIEP/C.I./003/2019).
Inclusion and Exclusion criteria: To be included, the students were men and women between 18 and 30 years of age. There were no restrictions on number of classes the participant was taking, if the participant was employed, their living conditions (living with family, friends, or alone), or marital status. Any participant was excluded if they were having a chronic disease, carrying out a specialized diet or taking some medication, or if, for women, were pregnant. From the 177 students who agreed to participate, 144 met the inclusion/exclusion criteria.
Sampling: The sample size was calculated assuming 40 to 60% of the Mexican population should have a FH-T2D20; therefore, we expected a 1:1 ratio. The sample size was calculated using: n=(NZ2p(1-p))/(e2(N-1)+Z2p(1-p)), where n=sample size, N=population size, p=probability of occurrence, Z=confidence level critical value, and e=maximum estimate error. The population of students enrolled in the Facultad de Medicina is around 6000. A sample size of at least 126 (63 per a group) was determined using the following assumptions: N=6000, 95% confidence interval (Z=1.96), e=10%, and p=0.5. Assuming a 70% class participation rate with a 30% dropout/incorrect data rate, the goal sample size was calculated to be 234. Using cluster sampling and the goal sample size, 7 classes were asked to participate in the study.
Study Variables
Anthropometric characterization: All anthropometric measurements were carried out in the morning between 8:00 and 10:00 am.
Weight (kg): The measurement was performed using a previously calibrated BAME scale with a 90cm stadiometer for 140kg. The measurement was made without shoes and wearing light clothing, and having an empty bladder, at least two hours after consuming food. The student was placed in the center of the scale ensuring that the weight was evenly distributed.
Height (cm): The measurement was performed after taking the weight and without getting off the scale. The head was placed in the horizontal Frankfort plane. The participant was asked to take a deep breath, hold, and maintain an upright posture while the movable base was brought to the top of the head with enough pressure to compress the hair.
BMI (kg/m2): The measurement was obtained by dividing weight by height squared. The value was interpreted as follows: "Anorexic" (<18.4kg/m2), "Normal weight" (18.5-24.9kg/m2), "Overweight" (25.0-29.9kg/m2), and "Obese" (30.0-39.9kg/m2).
Waist circumference (WC, cm): The measurement was determined using a HERGOM non-stretchable fiberglass tape measure (precision=1mm) at the narrowest part of the torso or at the midpoint between the lower rib and the iliac crest.
Hip circumference (HC, cm): The measurement was determined using a HERGOM non-stretchable fiberglass tape measure (precision=1mm) at the maximum gluteal extension level.
Waist to hip ratio (WHR) and waist to height ratio (WHtR): WHR and WHtR were calculated by dividing WC by HC or the height, respectively.
Tricipital Skinfold (TSF, mm): TSF was measured on the posterior region of the arm, at the midpoint between the acromion and the olecranon according to the technique proposed by Lohman. The Slim Guide caliper (precision=1mm) was used. TSF was interpreted according to Frisancho as "Low" (≤5 percentile), "Under average" (5-15 percentile), "Average" (15-75 percentile), "Over average" (75-85 percentile), and "Excess" (>85 percentile)21.
Mid-Arm Muscle Area (MAMA, cm2): MAMA was calculated using the corrected equation by Heymsfield22. Mid-upper arm circumference (MUAC, cm) was measured with the technique proposed by Lohman23. For women, MAMA=[MUAC-(π*TSF)]2/4*π-6.5]. For men, MAMA=[MUAC-(π*TSF)]2/4*π-10]. MAMA was interpreted according to Frisancho21 as "Low" (≤5 percentile), "Under average" (5-15 percentile), "Average" (15-85 percentile), "Over average" (85-95 percentile), and "Excess" (>95 percentile).
Fat Arm Index (FAI): Using Fat Area of the Arm (FAA) and Arm Area (AA), FAI was calculated as FAI=FAA/AA*100, where AA=MUAC2/4π and FAA=AA-MAMA. FAI was interpreted according to Frisancho21 as "Low" (≤5 percentile), "Under average" (5-15 percentile), "Average" (15-85 percentile), "Over average" (85-95 percentile), and "Excess" (>95 percentile).
Diet Evaluation
Collecting of diet information: Each participant carried out a 7-day food diary, which included at least one 2-day weekend. Before filling out of the food diary, the participants were trained to identify and report homemade measures to improve the recording of the quantity of the food consumed. Additionally, the participants were asked for photographic documentation of the food that was consumed to verify that the amount reported was correct. The dietary evaluation format included a section for mealtimes and salt or sugar added. Once the food diaries were completed, the following analyses were performed: quantification of macro and micronutrients, diet adequacy, and assessment of consumption of food groups as well as meal time, and caloric profile.
Quantification of macro and micronutrients: Using an in-house nutritional software, which uses the data published by the Mexican Equivalent System and the USDA food composition tables, each homemade measure was converted into grams and the total kilocalories and daily consumed macro and micronutrients were calculated.
Diet Adequacy: Using an in-house nutritional software, the amount (grams) of protein, carbohydrates, fats and kilocalories were calculated for each participant's daily and total consumption. Afterwards, the participant's average consumption of macronutrients was compared to the Bourges recommendations for Mexicans (proteins: 67.24g, fats: 60.89g, and carbohydrates: 293.90g), whereas the average consumption for kilocalories were compared with the energy requirement obtained by the Harris-Benedict formula using a physical activity factor of 1.4 (2054kcal). Diet adequacy (percent) was calculated as actual intake/requirement amount*100. Where actual intake is the participant´s average consumption and requirement amount is the Bourges recommendation and the Harris-Benedict formula. The same procedure was performed for all micronutrients. The participant's diet adequacy was classified as "Insufficient" (<90%), "Sufficient" (90-110%), and "Excess" (>110%).
Assessment of Consumption of Food Groups: Using the in-house nutritional software, each food/meal item was categorized as either cereal, fruits and vegetables, oils and fat, legume, proteins and manufactures products and then sub-categorized according to USDA data base. Afterwards, weekly consumption was determined by summing daily values. The portion of the population that consumed at least one food item from each subcategorization was calculated.
Meal Time: Consumption times were classified into meal times (Breakfast, Lunch, Dinner, and Snacks). For each participant, the energy corresponding to that meal time was averaged over the 7 days.
Caloric profile: After the daily amount of energy from protein, carbohydrates, and fats were calculated for each participant, the 7-day period was average and the energy distribution from each macronutrient for the total caloric intake was compared to the suggested require amounts for the participant's age group, according to the criteria from Bourges for the Mexican population, NOM-015 for the prevention and treatment of T2D, and the prevention goals for chronic disease established by the FAO/WHO.
FH-T2D
Genogram: FH-T2D was documented using a three-generation family genogram. Information about the participant's parents, siblings, aunts/uncles, cousins, and grandparents were collected. Participants with a family member diagnosed with pre-diabetes or T2D in any of their generational lines were classified as having a positive FH-T2D [FH-T2D(+)]. When the pathology was not reported, the participants were classified as negative [FH-T2D(-)].
Statistical analysis
Data are presented frequencies, percentages, mean and standard deviations or SEM. Differences between groups were determined using Y2 for categorical data. For continuous data, the Kolmogorov-Smirnov test was used to determine the normality. For parametric data, T-test was used to determine a difference between groups or a reference value, whereas for non-parametric data, the Mann U test was used. For multiple group comparison, differences between groups were calculated using a two-way ANOVA with a post hoc Tukey multiple comparison test. P-values <0.05 (two-tailed) were considered statistically significant and calculated using the IBM SPSS statistics software, version 26 (SPSS Inc., an IBM company, Chicago, Illinois, United States).
RESULTS
Selection of participants
The student body for BUAP medical school consists of over 6000 students. Using cluster sampling and the goal sample size, 7 classes were selected to participate. This led to an initial possible sample of 242 students; however, only 177 students initially agreed to participate in this study. 19 students were removed for not providing complete data and 12 were lost for filling out the food diary incorrectly. One student was removed because they were classified as an anorexic and another was removed because they were preparing for a body building competition. This resulted in 144 students to be used in the final analysis. Selection of participants is detailed in Figure 1. The total consumption of total population was used to obtain the results.
The general characteristics of the participants are shown in Table 1. 79.2% (95%CI:72.5- 85.8) of the sample had a FH-T2D. Overall, a majority of the cohort were normal weight. When stratified by FH-T2D, only parameters that presented with a significant difference between the groups were associated with obesity [weight (p=0.042) and BMI (p=0.036)] and peripheral adiposity distribution [HC (p=0.036)], having the higher values for those with FH-T2D. There was no difference in the ratio of males and females (p=0.794).
Table 1. Characteristics of the study participants
| Category | Overall | FH-T2D(-) | FH-T2D(+) | p-valuea |
|---|---|---|---|---|
| Sample size (M/F) | 144 (56 / 88) | 30 (11 / 19) | 114 (45 / 69) | 0.794 |
| Age (years) | 22.2 ± 1.7 | 22.3 ± 1.8 | 22.2 ± 1.8 | 0.262 |
| Weight (kg) | 66.3 ± 13.1 | 63.0 ± 13.5 | 67.6 ± 12.9 | 0.042* |
| Height (m) | 1.6 ± 0.1 | 1.6 ± 0.1 | 1.6 ± 0.1 | 0.574 |
| BMI (kg/m2) | 24.6 ± 3.8 | 23.7 ± 3.8 | 25.0 ± 3.7 | 0.036* |
| Normal weight (%) | 57.0 (48.2-64.3) | 73.3 (57.5-89.2) | 52.7 (42.6-60.9) | |
| Overweight (%) | 29.6 (21.7-36.6) | 13.3 (1.2-25.5) | 33.9 (24.7-42.0) | |
| Obese (%) | 13.4 (7.7-18.7) | 13.3 (1.2-25.5) | 13.4 (6.9-19.4) | |
| Waist circumference (cm) | 82.2 ± 11.1 | 80.4 ± 11.9 | 82.9 ± 10.8 | 0.231 |
| Hip circumference (cm) | 96.7 ± 10.6 | 95.2 ± 7.3 | 97.1 ± 11.3 | 0.036* |
| WHtR | 0.50 ± 0.06 | 0.49 ± 0.06 | 0.50 ± 0.06 | 0.298 |
| WHR | 0.90 ± 0.6 | 0.84 ± 0.09 | 0.90 ± 0.70 | 0.233 |
BMI: Body-mass index; FH-T2D(-): Subjects with no family history of Type 2 Diabetes; FH-T2D(+): Subjects with family history of Type 2 Diabetes; WHtR: Waist-to-height ratio; WHR: Waist-to-hip ratio. Values are either percent (95% confidence interval) or average 6 standard deviation. a Differences between FH-T2D(-) and FH-T2D(+) groups were determined using either Student's T-test or Whitney's Mann U for numerical data and Y2 for categorical data. * Indicates a significance difference (p<0.05, two-tailed).
Muscle and fat mass status by FH-T2D
For muscle mass, evaluated by the MAMA index, half of the total sample contained normal muscle mass, followed by under average and low muscle mass for their age group (Table 2). Interestingly, when stratified by FH-T2D, the FH-T2D(+) group was more likely to have average muscle mass than the FH-T2D(-) group, even though this difference was not significant (p=0.218). Moreover, the rates for decreased muscle mass [32.1% (95%CI:23.9-41.0) vs. 46.7% (95%CI:28.81-64.5)] and elevated muscle mass [11.6% (95%CI:5.5-17.2) vs. 16.6% (95%CI:3.3-30), respectively] were also not significant (p=0.421). This suggests that, for young, adult Mexicans, they are more likely to present with low or average muscle mass.
Table 2. Body fat and muscle mass classification.
| Category | Overall | FH-T2D(-) | FH-T2D(+) | p-valuea |
|---|---|---|---|---|
| MAMA (Muscularity) (cm2) | 35.5 ± 12.3 | 33.6 ± 12.7 | 36.0 ± 12.1 | 0.218 |
| Low (%) | 12.7 (7.1-17.9) | 16.7 (3.3-30.0) | 11.6 (6.9-19.4) | 0.421 |
| Under average (%) | 22.5 (15.4-29.0) | 30.0 (13.6-46.4) | 20.5 (12.8-27.5) | |
| Average (%) | 52.1 (43.2-59.5) | 36.7 (22.5-57.5) | 56.3 (46.1-64.4) | |
| Over average (%) | 3.5 (0.5-6.5) | 3.3 (0.0-9.8) | 3.6 (0.1-6.9) | |
| Excess (%) | 9.2 (4.3-13.7) | 13.3 (0.0-20.7) | 8.0 (2.9-12.8) | |
| TSF (Fat mass) (mm) | 19.2 ± 8.8 | 18.2 ± 5.2 | 19.5 ± 9.1 | 0.236 |
| Low (%) | 0.7 (0.0-2.0) | 0.0 (0.0-0.0) | 0.9 (0.0-2.6) | 0.883 |
| Under Average (%) | 12.7 (7.1-17.9) | 16.7 (3.3-30.0) | 11.6 (5.6-17.2) | |
| Average (%) | 62.0 (53.1-69.1) | 56.7 (38.9-74.4) | 64.3 (55.2-72.8) | |
| Over Average (%) | 7.7 (3.3-12.0) | 10.0 (0.0-20.7) | 7.1 (2.3-11.7) | |
| Excess (%) | 16.9 (10.6-22.7) | 16.7 (3.3-30.0) | 17.0 (9.8-23.5) | |
| FAI (Fat mass) | 48.4 ± 9.2 | 47.4 ± 9.5 | 48.8 ± 9.1 | 0.407 |
| Low (%) | 0.7 (0.0-2.0) | 3.3 (0.0-9.8) | 0.0 (0.0-0.0) | 0.298 |
| Under average (%) | 0.7 (0.0-2.0) | 0.0 (0.0-0.0) | 0.9 (0.0-2.6) | |
| Average (%) | 40.1 (31.6-47.6) | 46.7 (28.81-64.5) | 38.4 (28.8-46.6) | |
| Over average (%) | 12.7 (7.1-17.9) | 10.0 (0.0-20.7) | 13.4 (6.9-19.4) | |
| Excess (%) | 45.8 (37.0-53.3) | 40.0 (22.5-57.5) | 47.3 (39.1-57.4) |
MAMA: Mid-arm muscle area; TSF: Tricipital skinfold; FAI: Fat arm index; FH-T2D(-): Subjects with no family history of Type 2 Diabetes; FH-T2D(+): Subjects with family history of Type 2 Diabetes. Values are either percent (95% confidence interval) or average 6 standard deviation. a Differences between FH-T2D(-) and FH-T2D(+) groups were determined using either Student's T-test or Whitney's Mann U for numerical data and Y2 for categorical data.* Indicates a significance difference (p<0.05, two-tailed).
For adiposity, evaluated by the TSF index, overall, we found that most of the population had normal adiposity. When stratified by FH-T2D, the FH-T2D(+) group presented with a higher rate for normal fat mass than the FH-T2D(-) group (Table 2). Within the FH-T2D(+) group, there were more of participants with higher levels of adiposity than low adiposity [24.1% (95%CI:15.9-31.5) vs. 12.5% (95%CI:6.2-18.3) respectively]. Interestingly, the FH-T2D(-) group had a similar result [26.7% (95%CI:10.8-42.5) vs. 16.7% (95%CI:3.3-30.0), respectively]. However, when adiposity was measured as a function of arm fat, evaluated by the FAI index, 58.1% (95%CI:50.2-66.4) of the sample had elevated fat mass. When stratified by FH-T2D, again, with the FH-T2D(+) group, there were more participants with higher levels of adiposity than low adiposity [60.7% (95%CI:51.5-69.5) vs. 0.9% (95%CI:0.0-2.6), respectively] as well as with the FH-T2D(-) group [50.0% (95%CI:32.11-67.9) vs. 3.3% (95%CI:0.0-9.8), respectively]. Even though, most of the participants presented with normal muscle mass, a substantial number presented with an excess of adiposity.
Evaluation of dietary intake by food groups and mealtime
Using a 7-day food diary, we determined the quantity and rate of food consumption for the type of food within each food group consumed (see Table AM1 in Additional Materials: http://www.renhyd.org/index.php/renhyd/article/view/1090/665). With respect to Cereals (Figure 2A), Oils and Nuts (Figure 2B), Fruits and Vegetables (Figure 2C), Legumes (Figure 2D), Meats, Fishes, and Poultry (Figure 2E), and Manufacture products (Figure 2F), there was no difference in the intake between the FH-T2D(+) group and the FH-T2D(-) group. With respect to food consumption times (breakfast, lunch, dinner, or late-night snacking), both groups appear to consume most of their dietary intake during their snacking period (Figure 2G). For the FH-T2D(-) group, the distribution of food intake was not consistent between each consumption. However, for the FH-T2D(+) group, most participants received most of their food intake during their snack period, followed by dinner. Thus, it appears that, even though there is no difference in the types of food consumed, there is a difference when the food is consumed.

Using a 7-day food diary, we calculate the portion of the population with a FH-T2D (FH-T2D(+) (black bars) and without a FH-T2D (FH-T2D(-) (white bars) that consumed at least 1 portion of A) Cereals, B) Oils and Nuts, C) Fruits and Vegetables, D) Legumes, E) Meats, Fishes, and Poultry, and F) Manufacture products. Differences between groups was calculated using the Y2 test and significant differences (p<0.05) are indicated with *. G) Consumption times were classified into meal times (Breakfast, Lunch, Dinner, and Snacks) and for each participant the meal time' portion of the total energy was calculated. The bar height represents the average and the error bar represents the standard deviation. Difference between meal times was calculated using two-way ANOVA with a post hoc Tukey multiple comparison test. A significant result from Breakfast (p<0.05, two-tailed) is indicated with an *
Figure 2. Dietary patterns stratified by family history of Type 2 Diabetes (FH-T2D).
Energy distribution and caloric intake
The analysis of caloric intake demonstrated no difference between the FH-T2D(+) group and the FH-T2D(-) group (1673.2±504.1kcal vs. 1634.7±479.6kcal, respectively; p=0.778), (Figure 3A). Additionally, we determined the caloric value for the macronutrients provide, proteins (Figure 3B), carbohydrates (Figure 3C), and lipids (Figure 3D), and compared these results to the Bourges recommendations for the Mexican population24, the NOM-015 for preventions and treatment of diabetes25, and the FAO/WHO for the prevention of chronic diseases26. Both groups had a statistically significant low energy intake when compared with the recommendations by Bourges and the FAO/WHO; however, when compared with the recommendations of the NOM-015, the FH-T2D(+) group had a significant high energy intake (Figure 3A). Regarding the caloric input from each macronutrient, it was found that both groups significantly exceed the recommended energy from proteins and lipids (p<0.0001). While for carbohydrates, both groups failed to achieve the recommended levels but only the FH-T2D(+) group was the result significant (p<0.0001).

The total energy intake (A), proteins (B), carbohydrates (C), and lipids (D) were calculated for each participant using a 7-day food diary and stratified by family history of Type 2 Diabetes (FH-T2D). The average value for participants without FH-T2D (FH-T2D(-) (white bar), participants with FH-T2D (FH-T2D(+) (black bar) were compared to FAO/WHO (striped bar), NOM-015 (gray bar), and Bourges (checkered bar). Differences between FH-T2D groups and recommendations were calculated using a one sample T-test and a significant difference (p<0.05, two-tailed) was indicated with * vs. FH-T2D(-) and ** vs. FH-T2D(+). Bar length corresponds to the average.
Figure 3 Kilocalories and Caloric profile of macronutrients.
For the FH-T2D(+) and the FH-T2D(-) group, most of the participants consumed a hypocaloric diet [64.6% (95%CI:56.7-72.4)] (Table 3). Regarding protein intake, the greatest percentage of participants were found to have a hyperproteic diet. With respect to lipid consumption, we found that most of the FH-T2D(+) group did consume an insufficient amount of lipids, whereas the most of the FH-T2D(-) group did consume an adequate amount. With respect to carbohydrate consumption, we found the greatest percentage of participants did not consume a sufficient amount.
Table 3. Diet Adequacy Percentage determined by macronutrients and energy according to the Bourges criteria.
| Category | FH-T2D(-) | FH-T2D(+) | ||
|---|---|---|---|---|
| n (%: 95%CI) a | Mean±SD b | n (%: 95%CI) a | Mean±SD b | |
| Total energy (kcal) | ||||
| Insufficient (<1660) | 17 (56.7: 38.9-74.4) | 1317 ± 200 | 73 (64.0: 55.2-72.8) | 1400 ± 438 |
| Adequate (1660-2030) | 9 (30.0: 13.6-46.4) | 1850 ± 103 | 25 (21.9: 14.3-29.5) | 1880 ± 541 |
| Excess (>2030) | 4 (13.3: 1.2-25.5) | 2721 ± 546 | 16 (14.0: 7.7-20.4) | 2520 ± 532 |
| Protein (g) | ||||
| Insufficient (<60) | 6 (20.0: 5.7-34.3) | 54.0 ± 9.8 | 32 (28.1: 19.8-36.3) | 53.7 ± 10.4 |
| Adequate (60-76) | 10 (33.3: 16.5-50.2) | 75.2 ± 20.1 | 38 (33.3: 24.7-42.0) | 72.4 ± 26.1 |
| Excess (>76) | 14 (46.7: 28.8-64.5) | 85.7 ± 23.3 | 44 (38.6: 29.7-47.5) | 82.9 ± 17.0 |
| Lipids (g) | ||||
| Insufficient (<55) | 5 (16.7: 3.3-30.0) | 45.7 ± 8.4 | 49 (43.0: 33.9-52.1) | 46.9 ± 7.3 |
| Adequate (55-67) | 13 (43.3: 25.6-61.1) | 63.3 ± 7.9 | 30 (26.3: 18.2-34.4) | 65.7 ± 18.5 |
| Excess (>67) | 12 (40.0: 22.5-57.5) | 85.3 ± 27.4 | 35 (30.7: 22.2-39.2) | 83.6 ± 38.4 |
| Carbohydrates (g) | ||||
| Insufficient (<264) | 25 (83.3: 70.0-96.7) | 180.5 ± 41.7 | 96 (84.2: 77.5-90.9) | 186.8 ± 42.2 |
| Adequate (264-323) | 1 (3.3: 0.0-9.8) | 306.8 | 9 (7.9: 2.9-12.8) | 289.7 ± 14.7 |
| Excess (>323) | 4 (13.3: 1.2-25.5) | 396.5 ± 95.3 | 9 (7.9: 2.9-12.8) | 373.6 ± 51.0 |
95%CI: 95% confidence interval; FH-T2D(-): Subjects with no family history of Type 2 Diabetes; FH-T2D(+): Subjects with family history of Type 2 Diabetes; SD: Standard deviation. Values are either frequency (percent and 95% confidence interval) or mean 6 SD. a Differences between groups were determined using Y2 test. * indicates a significance difference (p<0.05, two-tailed). b Differences between groups were determined using Student's T-test or Whitney's Mann U test. * indicates a significance difference (p<0.05, two-tailed).
Low micronutrients consumption
Regarding the consumption of vitamins associated with the development of T2D, there was no significant difference between the FH-T2D(-) and FH-T2D(+) groups for the intake of vitamin D (p=0.152, Figure 4A) and vitamin E (p=0.649, Figure 4B); however, for vitamin A, the FH-T2D(+) group did consume less (p=0.034, Figure 4C). With regard to minerals that are associated with T2D development, magnesium (p=0.093, Figure 4D) and zinc (Figure 4E) intake were similar between the 2 groups. It should be noted that zinc intake, a mineral strongly associated with the prevention of T2D development, was almost significantly higher (p=0.051) in the FH-T2D(-) group. Interestingly, only for vitamin B12 (Figure 4F) did the participants reached levels near the recommended amount, whereas the other vitamins and minerals were well below the recommendations. For a full list, please see Table AM2 in Additional Materials (http://www.renhyd.org/index.php/renhyd/article/view/1090/665).
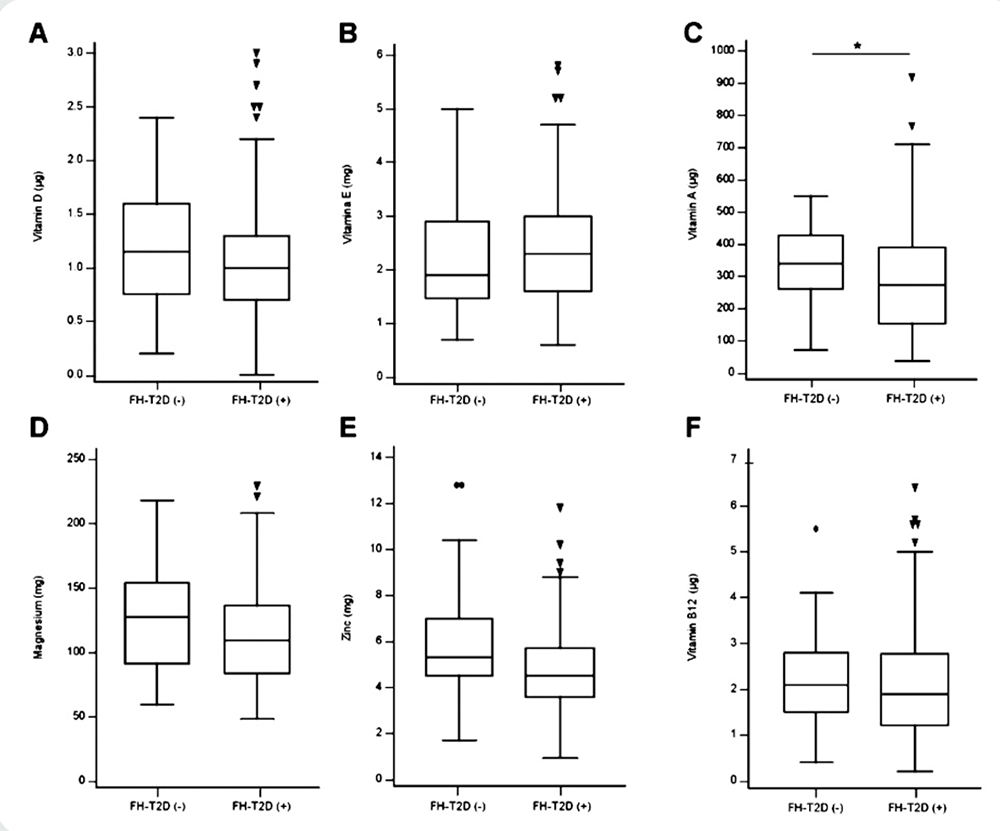
Using a 7-day food diary, Vitamin A (A), Vitamin D (B), Vitamin E (C), Vitamin B12 (D), Magnesium (E), and Zinc (F) were determined for participants without family history of Type 2 Diabetes [FH-T2D(-)] and participant with family history of Type 2 Diabetes [FH-T2D(+)]. Difference between groups were determined using the Mann U test and an * indicates a significant result (p<0.05).
Figure 4. Selected dietary vitamins and minerals intake for Medical students.
DISCUSSION
Here, we determined that a majority of medical students were not consuming food under ideal dietary patterns nor fulfilling the required nutritional status of key components of a balanced diet, according to the criteria from Bourges for the Mexican population, NOM-015 for the prevention and treatment of T2D, and the prevention goals for chronic disease established by the FAO/WHO. Moreover, when FH-T2D was taken into consideration, a fundamental difference between the 2 groups was observed.
In Mexico, 10.3% of the population suffers from T2D27 and Gomez-Landeros et al. has shown that 4 out of 10 Mexican students have a FH-T2D28. Moreover, according to ENSANUT, 34.8% of the non-diabetic population and 54.5% of the diabetic population have a FH-T2D20, which lead us to believe that, using a random sampling method (cluster sampling), 40 and 60% of our cohort would have a member with T2D. However, 79.2% of the cohort had a family member with T2D. This would suggest that the risk of developing T2D is increasing among the Mexican population and it is becoming detrimental for earlier interventions, such as lifestyle and diet counseling.
In this study, we examined the body's muscle and fat composition. We found that, independent of FH-T2D, both groups had a tendency to have decreased muscle mass. 30-40% of the cohort had below average muscle mass for their age group and sex. This suggests a sedentary lifestyle or that the body is in a stressful situation, in which the use of proteins as an energy source is promoted.
In support of this, many studies have shown that medical students fulfill these caveats29-32, thus it is possible that diets with increase protein consumption could mitigate this effect33. With respect to fat mass, we found that, in both groups, the highest prevalence was for average fat mass. However, what was disconcerting was that few had below average and most of the remaining participants had elevated fat mass. Depending on the index used, TSF versus FAI, the excess group consisted of 20-40% of the study population. Therefore, it is within reason to speculate that diet and lifestyle are promoting fat accumulation at the expense of protein in our cohort.
As expressed above, dietary patterns significantly affect muscle and fat mass accumulation34. Regarding dietary patterns, this study found a low consumption of fruits, vegetables, legumes, which can result in a diet low in fiber, vitamins, and minerals. We observed that, independent of FH-T2D, none of the participants' diet resulted in a sufficient consumption of key vitamins and minerals. Even though the FH-T2D(-) group consumed more vegetables, it was not enough to change their dietary pattern.
The need for the consumption of sweet foods has been related to a low insulin sensitivity or increased insulin resistance35. In our population, there was a notable higher consumption of sweets and bread for the FH-T2D(+) group compared to FH-T2D(-) group. In Mexico, the most consumed food was tortillas (in Central Mexican cooking, a tortilla is a thin pancake made of cornmeal, water, and calcium oxide, which can be filled with meats, vegetables, etc.); nevertheless, we still observed a low consumption of integral cereals and pastas made with wheat (spaghetti and noodles) in the FH-T2D(+) group. Fiber has many health benefits, such as lowering postprandial glucose, regulation of plasma cholesterol levels, preventing constipation, benefiting gut microbiota, among others36-38. Therefore, we posit that consumption of fiber within the recommendations (25-30g/day) would help to prevent the development of chronic diseases in the future39. In our study population, the intake of foods high in fiber, such as fruits, whole grains and legumes, was found to be decreased and even 20-30% of the students did not consume legumes. This is confirmed by the fact that these students also showed a deficiency for the consumption of vitamins and minerals. Therefore, these characteristics, over time, can provide the correct medium for the development of chronic diseases, such as diabetes.
A dietary pattern component of great significance are oils and fats. The analysis of oils and fats consumption reported that the most consumed category were fats and oils used for cooking as well as avocado oil. Other studies also coincide in a high consumption of fats for students of both animal origin and vegetable origin12,40. However, in none of these studies is the consumption of nuts and seeds discussed. In our population, the consumption of these items were very poor, and considering that they are a good source of mono- and poly-unsaturated fatty acids as well as protein and fiber41, it would be important for these foods to form part of the students' regular diet, maybe replacing unhealthy food items used as snacks. Even for patients with T2D, walnut consumption is suggested as a good source of vegetable proteins as well as an essential oil42. Therefore, as proposed by other researchers, it is suggested that students with a FH-T2D to increase the consumption of walnuts as a preventive measure for the development of T2D43.
Lastly, the type and time in which the food is consumed can affect the way that our body utilizes the nutrients44. When the data was analyzed by the consumption schedule, we observed that the FH-T2D(-) group tend to consume their food in the afternoon and at night snacks. This would postulate that they are maintaining a more regular schedule for food intake. However, for the FH-T2D(+) group, they consumed their food at night. When night consumption and late-night snacking is the major source of energy, studies have shown that this leads to more energy stores in the form of fat mass and this couple with stress could promote the use of protein as an energy source, leading to body muscle loss45. Interestingly, neither group conform to the standards suggested by NOM-015, Bourges, and FAO/WHO, indicating that none of the groups carried out a balanced diet. This failure is further confirmed by the inadequateness of the diet to contain proteins, lipids, and carbohydrates. The problem of not carrying out a balanced diet is that obtaining energy for the basic needs of the body would not be obtained from carbohydrates, whose main function is to provide energy, proteins or fatty acids would then be used. This fact added to the fact that the university population studying medicine is in constant academic-induced stress46, with a high demand for glucose due to mental activity47 and few hours of sleep48, this could explain the low muscularity and high prevalence of overweight found in our study population despite having a diet high in protein and low in calories.
This study has a few limitations. First, this is a cross-sectional study and any causal relationships cannot be confirmed, future prospective studies are currently underway. Second, the sample size of the FH-T2D(-) group is considerably small. Third, the percentile tables used to assess the population are based on Caucasian and other non-Mexican populations. Therefore, it is possible that for Mexico, the data could under-estimate the portion of subjects that were average. Fourth, despite the fact that this study tried to minimize consumption bias, the information on food intake is left to the decision of the participants. What is reported in the 7-day diary may present an overestimation or underestimation of their typical dietary life and energy consumption, due to their school-induced stresses. To minimize this effect, it would be beneficial to collect at multiple phases during their school life. However, upon initial assessment of the research protocol, we found that most students would have not participated. Lastly, alcohol consumption can increase the energy intake, as it provides 7 kilocalories per gram of alcohol. This means that when it is consumed in excess, that energy will also accumulate in the adipose tissue. Data about alcohol consumption was collected; however, it was not included in the results of this study as a sub-category because alcohol consumption was low among the students, most likely due to the students' academic responsibilities. Since alcohol consumption during university life is dependent on social interaction, we consider that alcohol consumption is more effective to be studied as a lifestyle rather than a part of student's diet. Nevertheless, alcohol consumption can underestimate the total caloric intake among the students as well as the fourth limitation.
Conclusions
Our study showed that for Mexican medical students, 77% were not consuming a proper diet, which was confirmed when compared to the standard recommendations for Mexicans that are associated with prevention of T2D development. Furthermore, there was a tendency for low muscle mass and an increase in fat mass among Mexican medical students, regardless of FH-T2D. As our study suggests, this could be due to poor eating habits including the time and type of food consumed, low consumption of legumes, whole grains, and a diet low in essential fatty acids. Therefore, if the nutritional recommendations are not followed, those with a FH-T2D could develop the disease at an earlier age.















