My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Ars Pharmaceutica (Internet)
On-line version ISSN 2340-9894
Ars Pharm vol.57 n.2 Granada Apr./Jun. 2016
https://dx.doi.org/10.30827/ars.v57i2.4987
ORIGINAL ARTICLE
Miconazole Microsponges based topical delivery system for diaper dermatitis
Sistema de administración tópica basada en microesponjas de miconazol para la dermatitis del pañal
Neha Gulati1, Neha Tomar2 and Upendra Nagaich1
1 Department of Pharmaceutics, Amity Institute of Pharmacy, Amity University, Noida (U.P.), India;
2 Department of Pharmaceutics, Bharat Institute of Technology, Meerut (U.P.), India
ABSTRACT
Aim: The current investigation was aimed to develop and optimize the microsponges of miconazole nitrate for treatment of diaper dermatitis for enhanced therapeutic effect.
Material and Methods: Microsponges were developed by emulsion solvent diffusion technique using 23 factorial design. Fabricated microsponges were optimized in order to analyze the effects of independent variables on the encapsulation efficiency, particle size, surface topography and in vitro drug release. The optimized formulation was then incorporated into the gel and evaluated.
Results: Particle size of all formulations was found to be uniform and scanning electron microscopy (SEM) indicates spherical shape and porous nature of microsponges. In vitro drug release studies of all formulations revealed the release rate within the range of 67%±0.09 to 80.6%± 0.68 at the end of 12 hours. On its basis, formulation F8 was selected and incorporated into the gel (CF8) which was evaluated for pH, viscosity, spreadability, in vitro drug diffusion studies, in vitro anti fungal studies and stability studies.
Conclusion: The formulated microsponge-based gel of miconazole nitrate would be a capable substitute to traditional treatment for reliable and economical cure of diaper dermatitis.
Key Words: Microsponge delivery system, Eudragit RS100, Miconazole Nitrate, Topical Drug Delivery, yeast diaper dermatitis.
RESUMEN
Objetivo: La presente investigación tuvo como objetivo desarrollar y optimizar las microesponjas de nitrato de miconazol para el tratamiento de la dermatitis del pañal para un efecto terapéutico mejorado.
Material y métodos: Las microesponjas fueron desarrollados por emulsión técnica de difusión del disolvente usando un diseño factorial 23. Las microesponjas fabricadas han sido optimizadas con el fin de analizar los efectos de las variables independientes sobre la eficacia de encapsulación, tamaño de partícula, la topografía de la superficie y en la liberación de fármaco in vitro. A continuación, la formulación optimizada se incorporo en un gel y se evaluó.
Resultados: Se encontró que el tamaño de partículas de todas las formulaciones fue uniforme y la microscopía electrónica de barrido (SEM) indicó forma esférica y de naturaleza porosa de las microesponjas. En la liberación del fármaco, estudios in vitro de todas las formulaciones, revelaron la velocidad de liberación dentro de un intervalo de 67±0,09% a 80,6±0,68% al cabo de 12 horas. Con esta base, La formulación F8 se seleccionó y se incorporó en el gel (CF8) en el que se evaluó el pH, viscosidad, capacidad de extensión, estudios de difusión in vitro del fármaco, estudios in vitro anti hongos y estudios de estabilidad.
Conclusión: El gel a base de microesponja formulado de nitrato de miconazol sería un sustituto adecuado al tratamiento tradicional para la curación fiable y económica de la dermatitis del pañal.
Palabras clave: Microsponge, Liberación de fármacos, Eudragit RS100, Nitrato de miconazol, Dermatitis del pañal.
Introduction
Numerous drug delivery systems have been developed for the drugs as transdermal delivery system (TDS) using the skin as doorway for entrance. Despite of improved efficacy of drug delivery, TDS is not realistic for the delivery of actives whose final target is skin itself[1]. Drug controlled release onto epidermis asserts that the drug remains primarily localized and does not enter the systemic circulation in significant amounts. Many conventional formulations such as ointments, gels etc. require high concentrations of drug for an effective therapy, which results in great number of side effects. Thus, it becomes essential to boost the amount of time that a drug remains on skin surface or epidermis and simultaneously decreasing its transdermal penetration into the body[2].
The microsponge delivery system (MDS) satisfies these constraints. MDS is a distinctive technology comprises of 10-100um diameter drug loaded microporous beads which aids in controlled delivery of drugs[3]. Rubbing, moisture, pH, friction, or ambient skin temperature are triggering factors for the controlled release of drug from microsponges by diffusion through skin. Microsponges has the facility to load wide range of active ingredients like essential oils, fragrances, anti infectives, antifungals, etc[4].
Topical fungal infections are among the most common skin disorders and occur in both healthy and immune compromised persons. Dermatophytes, yeasts and non-dermatophyte molds cause them. For the new borns and toddlers, diaper rash or yeast diaper dermatitis is a widespread problem which develops on the bums[5]. Yeast is a fungus that lives on the skin and in the intestines, and when it gets a warm and moist environment in the diaper area, it causes rash. To treat these rashes, antifungal drugs loaded creams and ointments are prescribed which have problems of conventional topical drug delivery system like high dose for effective therapy and high dosing frequency[6].
Miconazole nitrate provides relief from the symptoms of diaper dermatitis. Infants having more than 4 weeks of age are given maximum treatment up to 7 days followed by application of lubricants or emollients as protective layer[7]. Miconazole Nitrate inhibits ergosterol synthesis that results in increased cellular permeability, causing leakage of cellular contents. Other antifungal effects of azole compounds have been proposed and include inhibition of endogenous respiration, interaction with membrane phospholipids, and inhibition of yeast transformation to mycelial forms[8].
Thus, the aim of present investigation is to develop miconazole nitrate loaded microsponges delivery system with an aim to reduce the dose and dosing frequency associated with conventional topical drug delivery system and achieve the therapeutic objective. The fabricated microsponges will be characterized with respect to particle size, surface morphology, drug entrapment efficiency, in vitro diffusion studies, in vitro antifungal studies, release kinetics and stability studies.
Material and Methods
Materials
Miconazole nitrate was a kind gift from Basic Pharma Life Sciences Pvt. Ltd., Gujarat. Eudragit RS 100, Polyvinyl Alcohol, Dichloromethane and ethanol was purchased from S.D fine Chem Ltd. All solvents are of analytical grade.
Methodology
Statistical Experimental Design for formulation Optimization
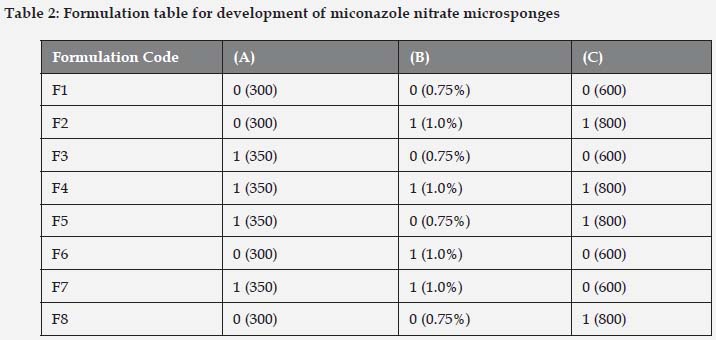
A full factorial design consists of two or more factors, each with discrete possible values or "levels", and whose experimental units take on all possible combinations of these levels across all such factors. A common experimental design is one with all input factors set at two levels each. These levels are called 'high' and 'low' or '1' and '0', respectively. A design with all possible high/low combinations of all the input factors is called a full factorial design in two levels[9, 10]. If there are k factors, each at 2 levels, a full factorial design has 2k Factorial design. 23 imply 8 runs as shown in Table 1.
Preparation of Miconazole nitrate loaded Microsponges
Microsponges were prepared by quasi emulsion solvent diffusion as shown in Table 2. The required amount of miconazole nitrate and Eudragit RS100 polymer were weighed accurately and dissolved in 5ml of Dichloromethane: Ethanol (1:1) under sonication. Polyvinyl alcohol was weighed accurately and dissolved in distilled water at 60oC. The surfactant was allowed to cool to room temperature. The internal phase containing miconazole nitrate and eudragit RS100 was added drop wise with the aid of syringe with stirring at 800 rpm until the complete diffusion of the external phase i.e. about 8 h. After complete diffusion of the external phase, the microsponges were filtered and dried overnight at room temperature as shown in Figure 1.[11, 12]
Characterization of Miconazole nitrate microsponges
Particle Size and Size Distribution Analysis
The particle size was determined using an optical microscope. The microscope was fitted with a stage micrometer to calibrate the eyepiece micrometer[13]. The values were given for the formulations in the form of mean particle size range.
One division of stage micrometer = 0.01 mm = 10 μm.
C = SM × 10/EM
Where,
C = correction factor;
SM = reading of stage micrometer which coincides with reading of eyepiece micrometer (EM).
The particle diameter of around 100 particles was measured.
The average particle size was determined using the following formula:
Dmean = Σnd/ Σn
Where, n = number of microsponges observed and
d = mean size range.
Each formulation was observed three times and average of three trials was calculated.
Shape and Surface Morphology of Microsponges
Shape and surface morphology of miconazole microsponges was visualized by scanning electron microscopy (LEO-430 Cambridge and U.K). Samples were prepared by lightly sprinkling microsponges on a double adhesive tape, on an aluminum stub. The stubs were then coated with gold to a thickness of 200 to 500 A0 under an argon atmosphere using gold sputter module in a high vacuum evaporator[14]. The samples were then randomly scanned and photomicrographs taken at different magnifications with SEM.
Drug entrapment efficiency
The entrapment efficiency and drug loaded microsponges were estimated by dissolving 50 mg of microspheres in 0.01 N HCl. The samples were analyzed using UV-Visible spectrophotometer (Shimadzu UV 1800) at a wavelength 272 nm. Entrapment efficiency was calculated in triplicate using the following equations[15]:

Production Yield
For calculating production yield, theoretical mass was calculated initially by taking the weight of excipients. The production yield of the microsponges was determined accurately by calculating the initial weight of the excipients and the final weight of the formulation obtained[16]. Production Yield was calculated in triplicate using the following equation.

In Vitro Drug Release Studies
The drug release studies of miconazole nitrate loaded microsponges were carried out using basket dissolution apparatus (USP Type I apparatus). 900ml of fresh phosphate buffer saline (PBS pH 5.5) was used for the release studies. The temperature of the dissolution medium was controlled at 32±1oC with 150 rpm rotation speed. Microsponges equivalent to 50 mg of miconazole nitrate were weighed. At fixed time intervals, aliquots of samples were withdrawn and replaced by an equal volume of fresh dissolution medium to maintain the sink conditions. After suitable dilution, the samples were analyzed spectrophotometrically at 272 nm (Shimadzu UV-1208) against blank.
The release studies were carried out for 12 h.[17]. Based on the evaluation characteristics, the optimized formulation of each polymer was incorporated into suitable gel bases and evaluated further. All the readings were repeated three times.
Preparation of Miconazole Nitrate Microsponges loaded Topical gel
Gel formulation was developed using carbopol® 934 as gelling agent. The accurately weight quantity of carbopol® 934 (1%w/v) was dispersed in beakers containing adequate amount of water under constant stirring and allowed to hydrate for 24 h at room temperature. Later, glycerine and optimized microsponges formulation containing miconazole nitrate was incorporated into the carbopol gel with the help of mechanical stirrer at 25 rpm. Then, water was added to gel under constant stirring. The dispersion was neutralized using triethanolamine (0.5% w/w)[18]. The gel was allowed to stand overnight to remove entrapped air. The formulation was transferred to a suitable container and stored for further studies.
Evaluation of Microsponges Loaded Gel Formulations:
The prepared gels were evaluated for different parameters such as pH, appearance, viscosity, spreadability, drug content and drug release, in vitro antifungal activity and stability studies with the aim of checking the efficacy of microsponges loaded gel formulations.
Appearance
The prepared gel bases were inspected visually for clarity, colour and presence of any particles. Transparent gel was found in microsponges loaded carbopol gel.
pH
1g of microsponges loaded gel formulation was dissolved in 100 ml water and the pH was determined with the help of digital pH meter[19]. All the gels were tested for pH three times and average of three determinations was calculated.
Rheological Studies
Spreadability:
The spreadability studies of microsponges loaded gel formulations were carried out by keeping gel between two horizontal glass slides of standard dimension. 100 g weight was placed on top of the two slides so that the formulation gets uniformly spread. The weight was removed and excess formulation was scraped out[20]. The experiment was carried out in triplicate.
S = m. l/ t
Where,
m = weight kept on the upper slide
l = length of glass slide
t= time taken in seconds.
Viscosity:
Viscosity of microsponges loaded gel formulation was determined using Brookfield viscometer with spindle No. 6 at 10 rpm at temperature 37±0.5oC[21].
Drug Content
One gram of gel formulation containing drug equivalent to 10 mg of miconazole nitrate was extracted and the volume was made up to 50 ml with ethanol. The resulting solution was filtered. Suitable dilutions of the filtrate were prepared with filtered phosphate buffer pH 5.5 and absorbance was measured at 272 nm using UV spectrophotometer (Shimadzu UV-1208). All readings were taken in triplicate and average was calculated[22].
In vitro Drug Diffusion Studies
In vitro diffusion study was conducted using Keshary-Chien (K-C) cell with an effective diffusion area of 2.0 cm2 and a cell volume of 25 mL. Phosphate Buffer Saline (pH 5.5) was used as dissolution medium, and system was thermoregulated with a water jacket at 37±1oC under constant stirring. The cellophane membrane (0.45 µm) previously soaked overnight in dissolution medium was mounted onto K-C cell. Microsponges loaded gel formulation was assessed for diffusion study. 1ml of sample aliquots were withdrawn at predetermined time intervals and subsequently replenished with an equal amount of phosphate buffer. The samples were filtered, diluted and analyzed using UV spectrophotometer at 272nm against blank[23]. The release studies were carried out for 10 h and the release data were analyzed by means of diverse mathematical models to know release kinetics.
In vitro Antifungal Study
For antifungal studies, sabouraud's dextrose agar was utilized. The media was taken in a 250 ml conical flask and dissolved in 100 ml of distilled water. The pH was adjusted to 5.6 ± 0.2. The medium was sterilized in an autoclave at 15 lbs (121oC) for 15 min. After sterilization, the medium was allowed to cool down at room temperature and poured into presterilized petridishes inside a laminar airflow unit with layer of uniform thickness. Medium filled petridishes were kept in laminar airflow unit for solidification, after which a loop of diluted suspension culture (Candida albicans) in nutrient broth was added on to the surface of solidified agar and was spread uniformly with the help of spreader. Culture was then stabilized, subsequent to which 6mm diameter cups were punched using sterile cork borer and scraped out from the petridish[24].
Microsponges loaded gel formulation (equivalent to 0.25% miconazole nitrate) and marketed formulation of miconazole nitrate was fed into the cup separately. The petridishes were then incubated for 24 h at 37oC. After incubation the zone of inhibition was measured.
Release Kinetics
Data obtained from release studies of gels was subjected to kinetic treatment such as zero order drug release, first order drug release, Higuchi's square root plot and Korsmeyer-Peppas model to obtain the order of release and release mechanism[25].
Stability studies
Optimized gel formulation was subjected to stability testing as per ICH guidelines. Gel was filled in clean, glass vials and various replicates were kept at 5oC±2, 25oC±2 and 40oC/75% RH in a humidity chamber for a period of 60 days [26]. Gel was assessed for change in appearance and drug content at an interval of 7, 15, 30, 45 and 60 days.
Result and Discussion
Miconazole nitrate loaded microsponges were successfully formulated using quasi-emulsion solvent diffusion technique. The technique used for the fabrication is very simple, replicable and quick[11]. Eudragit RS 100 and polyvinyl alcohol were used as microsponges forming polymer and surfactant respectively. 23 factorial design was applied for the optimization of formulation parameters and process variables. Prior to application of factorial design, a broad range optimization was carried out so as to widely select the parameters for further experimentation.
Different concentrations of polymer viz. 100 to 400mg were chosen for its optimization and keeping other parameters constant like surfactant 0.75%w/v and 800 rpm stirring speed. Out of all concentration, 300mg polymeric concentration showed spherical porous microsponges with good entrapment efficiency 79.6%±0.23 and 66.7±0.17 µm particle size as shown in Figure 2. Thus, it was selected for further optimization. The type and concentration of surfactant has a key role to play in the preparation of microsponges. By keeping other parameters constant (polymer 300mg and stirring speed 800rpm) and varying the concentration of surfactant from 0.5% to 1.5 % w/v, the particle size also varied from 66.43μm to 92.54 μm. The minimum concentration of surfactant required to bring about the formation of uniform microsponges was found to be 0.75% w/v as illustrated in Figure 3. Stirring speed also played a crucial role in the formation of microsponges with reduced particle size. Likewise varying stirring speed was selected viz. 100, 400, 800, 1200 rpm and keeping other parameters (polymer 300mg and surfactant 0.75%w/v) constant. The results showed formulation prepared with 800 rpm stirring speed showed smallest particle size and good entrapment efficiency 65.25%±0.34 as shown in Figure 4. Thus, 300mg polymer concentration, 0.75%w/v surfactant concentration and 800rpm stirring speed were selected for the further optimization with the aid of factorial design.
The 23 factorial design enabled the selection of optimized formulation. Three factors (viz. polymer concentration, surfactant concentration and stirring speed) and two levels (low (0) & high (1)) were selected as shown in Table 1, as these are most important factors (independent variables), which can affect particle size, entrapment efficiency and in vitro drug release (dependent variables).
The mean particle size of microsponges was found to be in the range of 10.32μm±0.32 to 98.45±0.71μm. Visual assessment of all formulations was done using optical microscope, which clearly indicates that as the particle size increased with increase in the concentration of polymer. It may be due to additional presence of polymer in solution, which in turns increases, the wall thickness resulting larger microsponges. Furthermore, increasing amount of polymer causes increase in apparent viscosity at augmented polymeric concentrations. Consecutively, bigger emulsion droplets formed and size of microsponges increases. Formulation F8 showed minimum particle size 66.42± 0.31 µm which may be due to optimum concentration of polymer and surfactant. High stirring speed results in smallest particle size that is stabilized by surfactant.
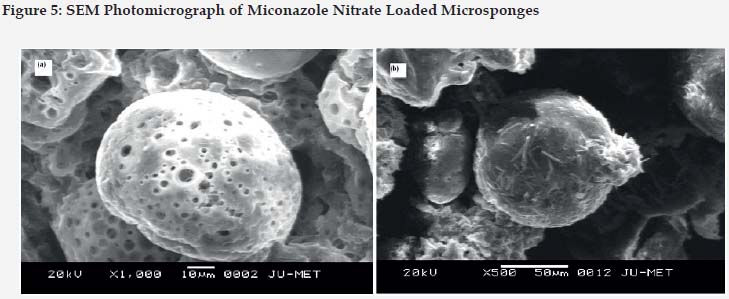
For morphology and surface topography investigation, fabricated microsponges were subjected to SEM analysis. The captured SEM image of microsponges is shown in Figure 5. It was observed that the obtained microsponges were substantially porous, predominantly spherical and have uniform outline. Highly Porous morphology may be attributed due to diffusion of solvent from microsponges surface. The findings of entrapment efficiency were found to be in the range of 50.18%± 0.23 to 83.5% ± 0.91 for all the eight formulations. Likewise, entrapment efficiency increases due to increase in polymer concentration. Highest entrapment was found to be 83.5% ± 0.91 which was shown by F8 formulation due to optimum polymer and surfactant concentration.
The production yield of all eight formulations ranged from 78.66%± 0.10 to 87.5%± 0.91. The polymer concentration was found to have a significant effect on production yield. Figure 6. High polymer concentration reduces the dichloromethane diffusion rate from viscous polymeric solution to aqueous phase that provides more time for droplet formation and thus yield improves.
In vitro drug release studies were successfully done using basket type apparatus and the cumulative percentage drug release at the end of 12 hrs was noted to be 67.5%±0.09 to 80.6%±0.68. Maximum release was shown by F8 formulation i.e. 80.6%±0.68. The cumulative release profiles displayed a bi-phasic release with an initial burst effect i.e. 3.8±0.12 in 0.25 h to 26.7±0.29 in 2h followed by sustained release as shown in Figure 7. Drug release decreases with an increase in polymer concentration.
On the basis of factorial design, miconazole nitrate microsponge formulation F8 was found to be optimized for further gel formulation. Gel was successfully formulated using 1%w/v carbopol® 934 as gelling agent, glycerin as smoothening agent and triethanolamine as cross-linking agent.
Gel was found transparent, uniform with no presence of air molecules. The pH values of all formulations were found in the range of 5.7-5.8, which are supposed to be suitable to pass up threat of nuisance on application to skin. The outcome of spreadability illustrated that formulated gels get easily spread on applying small amount of shear. Spreadability of gel containing microsponges was found to be 2.54g.cm/s; signifying that spreadability of drug loaded microsponge gel was good. The viscosity of prepared miconazole nitrate loaded microsponge gel was found to be 38,200 cPs. The viscosity was found to be dependent on polymeric content of formulation. In vitro diffusion studies of microsponges loaded gel was conducted in phosphate buffer saline (pH 6.8) using K-C cell upto 12 h. sAccording to the results obtained, it was noted that, the cumulative drug diffused at the end of 10 hrs was 82.78%± 0.91. Results of cumulative drug diffused were subjected to release kinetics models that portrayed that the formulation follows higuchi release (r2=0.982). To confirm the exact mechanism of drug release, the data were fitted according to Korsemeyer-Peppas equation. The value of N = 0.86 which shows non-fickian in nature as shown in Table 3. Optimized microsponges formulation (F8) loaded carbopol gel showed maximum zone of inhibition of 17mm as compared to marketed formulation (15mm) of miconazole nitrate.
Stability studies results of optimized microsponges formulation loaded carbopol gel displayed no significant changes in the physical appearance and drug content which clearly suggest that the formulations are stable at 5oC and 25oC. However, some drug degradation was observed at 40o C/ 75 % RH as tabulated in Table 4.
Conclusion
For the existing era and future prospects, sustained drug delivery by means of polymer based systems has been recommended to exist owing to frequent probable benefits for technical and cost effective grounds. The notion behind the development of polymer based microsponge delivery system was to release the active in a recurrent approach for wide time period to cut the dosing frequency and to improve bioavailability. The technique executed was emulsion solvent diffusion that was found to be simple, reproducible and rapid. Fabricated microsponges were spherical in shape and have porous texture. Microsponge based gel reflected Higuchi release kinetics and controlled by non-fickian release mechanism. Thus, gel comprising microsponges was found as an potential drug delivery system presenting prolonged release of miconazole nitrate in the treatment of diaper dermatitis.
Competing interest
Authors declared that there is no conflict of interest associated with this research work.
References
1. Bhanu VP, Shanmugam V, Lakshmi PK. Development and optimization of novel diclofenac emulgel for topical drug delivery. Int J Comp Pharm. 2011; 2, 1-4. [ Links ]
2. Osmani RA, Aloorkar NH, Kulkarni AS, Harkare BR, Bhosale RR. A new cornucopia in topical drug delivery: microsponge technology. Asian J Pharm Sci Technol. 2014; 4, 48-60. [ Links ]
3. D'souza JI, More HN. Topical anti-inflammatory gels of fluocinolone acetonide entrapped in eudragit based microsponge delivery system. Res. J. Pharm. Technol. 2008; 1, 502-506. [ Links ]
4. Vyas LK, Tapar KK, Laddha BH, Lahoti AO, Nema RK. Formulation and development of anti-blemish preparations using microsponge technology. J. Chem. Pharm. Res. 2010; 2, 562-571. [ Links ]
5. Morelli JG. Cutaneous fungal infections. In: Kliegman RM, Stanton BF, St. Geme JW III, et al., eds. Nelson Textbook of Pediatrics. 19th ed. Philadelphia, PA: Elsevier Saunders; 2011: 658. [ Links ]
6. Fernandes JD, Machado MCR, Prado de Oliveria ZN. Clinical presentation and treatment of diaper dermatitis-Part-II. An. Bras. Dermatol 2009.84(1): 47-54. [ Links ]
7. Concannon P, Gisoldi E, Phillips S, Grossman R. Diaper dermatitis: a therapeutic dilemma. Results of a doubleblind placebo controlled trail of miconazole nitrate 0.25%. Pediatr Dermatol. 2001;18:149-55. [ Links ]
8. Parashar B, Kabra A, Chandel A. Formulation and Evaluation of Gel Containing Miconazole Nitrate an Antifungal Agent. Int J Pharm Res Rev 2013; 2(6):18-28. [ Links ]
9. Lewis GA, Mathieu D, Phan-Tan-Luv R. Pharmaceutical experimental design. New York: Marcel Dekker; 1999. [ Links ]
10. Patel DM, Jani RH, Patel CN. Design and evaluation of colon targeted modified pulsincap delivery of 5-fluorouracil according to circadian rhythm. Int J Pharma Investig 2011;1:172-81. [ Links ]
11. Shelke OS, Sable KS, Gadhave MV, Gaikawad DD. Microsponge Drug Delivery System: An Emerging Tool for Topical Drug Delivery System. The Global J Pharm Res 2012;1(4):805-818. [ Links ]
12. Kawashima Y, Niwa T, Handa T, Takeuchi H, Iwamoto T, Itoh K, et al. Preparation of controlled-release microspheres of Ibufrofen with acrylic polymer by noval quasi-emulsion solvent solvent diffusion method. J Pharm Sci 1989; 78: 68-72. [ Links ]
13. Shaha V, Jain H, Krishna J, Patel P. Microsponge drug delivery: A review, Int J Res Pharm Sci, 2010; 1(2): 212-218. [ Links ]
14. Gulati N, Nagaich U, Saraf SA. Fabrication and in vitro characterization of polymeric nanoparticles for Parkinson's therapy: a novel approach. Braz. J Pharm. Sci. 2014;50(4):869-876. [ Links ]
15. Mahajan AG, Jagtap LS, Chaudhari AL, Swami SP, Mali PR. Formulation and evaluation of microsponge drug delivery using indomethacin, Int Res J Pharm, 2011; 2(10): 64-69. [ Links ]
16. Kilicarslan M, Baykara T. The effect of the drug/polymer ratio on the properties of verapamil hydrochloride loaded microspheres. Int. J. Pharm. 2003; 252, 99-109. [ Links ]
17. Saboji JK, Manvi FV, Gadad AP, Patel BD. Formulation and evaluation of ketoconazole microsponge gel by quasi emulsion solvent diffusion. J Cell Tissue Res. 11(1), 2011, 2691-2696. [ Links ]
18. Mayur K, Ramesh K, Nitin J, Prashant P, Rajendra G, Jeevan N, Ethyl cellulose based microsponge delivery system for antifungal vaginal gels of tioconazole, J Drug Deli Therap, 2013; 3(6), 14-20. [ Links ]
19. Jain V and Singh R. Development and characterization of Eudragit RS 100 loaded microsponges and its colonic delivery using natural polysaccharides. Acta Pol. Pharm. Drug Res. 2010; 67, 407-415. [ Links ]
20. Rao NGR, Rao KP and Muthalik S. Clinical studies and antimicrobial activity of ciprofloxacin hydrochloride medicated dental gels for periodontal infection. Asian J Pharm. 2009; 3(2): 125-134. [ Links ]
21. Loganathan V, Manimaran S, Sulaiman A, Reddy MVS, Senthil BK and Rajaseskar A. The effect of polymers and permeation enhancers of flubiprofen from gel formulations. Indian J Pharm Sci. 2001; 63(3): 200-204. [ Links ]
22. Hussain H, Dhyani A, Juyal D, Bahuguna A. Formulation and evaluation of gel-loaded microsponges of diclofenac sodium for topical delivery. The Pharma Innovation Journal 2014; 3(10): 58-63. [ Links ]
23. Rizkalla Z, Latif Aziz CM, Soliman R. In-vitro and invivo evaluation of hydroxyzine hydrochloride microsponges for topical delivery. AAPS Pharm. Sci. Technol. 2011; 12, 989-1001. [ Links ]
24. Shetgaonkar T, Narayana Charyulu R. Microsponge Drug Delivery of Terbinafine Hydrochloride for Topical Application. Int J Pharm Sci Rev Res. 2015;33(1):48-54. [ Links ]
25. Dash S, Murthy PN, Nath L, Chowdhury P, Kinetic modeling on drug release from controlled drug delivery systems. Acta Poloniae Pharmaceutica Drug Res. 2010; 67(3): 217-223. [ Links ]
26. Zhang Z, Liao G, Nagai T, Hou S. Mitoxantrone polybutylcy-anoacrylate nanoparticles as an anti-neoplastic targeting drug delivery system. Int J Pharm. 1996;139:1-8. [ Links ]
![]() Correspondence:
Correspondence:
Dr. Upendra Nagaich
Department of Pharmaceutics,
Amity Institute of Pharmacy,
Amity University,
Noida (U.P.), India
upendra_nagaich@hotmail.com
09301907999
Received: 18-04-2016
Accepted: 03-06-2016