My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Ars Pharmaceutica (Internet)
On-line version ISSN 2340-9894
Ars Pharm vol.57 n.3 Granada Jul./Sep. 2016
https://dx.doi.org/10.30827/ars.v57i3.5331
ORIGINAL ARTICLE
Development and Evaluation of Isoniazid Loaded Silk Fibroin Microsphere
Desarrollo y evaluación de microesfera de seda Fibroin cargado de isoniacida
Narinder Singh, Surya Prakash Gautam, Harjaskaran, Amanjot, Lovepreet Singh, Ankit Verma and Shalu Rani
Department of Pharmaceutics, CT institute of Pharmaceutical Sciences, Shahpur, Jalandhar. India
ABSTRACT
Aim: Current experimental investigation is dedicated to prepare microspheres with small size and good sphericity by Phase Separation method using Isoniazid (INH) as model drug. Silk fibroin has unique intrinsic qualities like biodegradability, biocompatibility or release properties and their tunable drug loading capacity. The delivery loading proficiency of the drug molecules in silk spheres be contingent on their charge, and hydrophobicity or subsequent in altered drug release profiles.
Methods: In the present work Isoniazid loaded silk fibroin microsphere was prepared by using phase separation method. Microsphere was evaluated for Ultraviolet-visible spectroscopy, Fourier Transform infrared spectroscopy, Entrapment efficiency, Scanning electron microscopy Studies.
Results: Scanning electron microscopy studies revealed that Isoniazid Loaded Silk Fibroin Microspheres were spherical. Entrapment Efficiency of Isoniazid loaded Microspheres of different Formulation from F1 to F5 was in range of 53 to 68 %. F3 showed 68.47 % entrapment Efficiency and the optimized formulation drug release was 93.56 % at 24 hours.
Conclusion: Experimental report disclosed a new aqueous based formulation method for silk spheres with controllable shape or size and sphere. Isoniazid loaded silk microspheres may act as ideal nano formulation with elaborated studies.
Keywords: silk fibroin; nanoformulation; biodegradability; isoniazid.
RESUMEN
Objetivo: La investigación experimental en curso está dedicada a la preparación de microesferas de pequeño tamaño y buena esfericidad mediante el método de separación de fases con isoniazida (INH) como fármaco modelo. La fibroina de seda tiene cualidades intrínsecas únicas como la biodegradabilidad, biocompatibilidad o propiedades de liberación y su capacidad de carga de fármacos ajustable. La aptitud de entrega de carga de las moléculas de fármaco en las esferas de seda estar supeditada a su carga, y la hidrofobicidad o subsiguiente alteración en los perfiles de liberación de fármacos.
Métodos: En el presente trabajo la microesfera de fibroina de seda cargada de isoniazida fue preparada utilizando el método de separación de fases. La microesfera fue evaluada por espectroscopia ultravioleta-visible, espectroscopia infrarroja con transformado de Fourier, se midió la eficiencia de atrapamiento y se estudios mediante microscopia electrónica de barrido.
Resultados: Estudios con el microscopio de escaneo de electrones revelaron que las microesferas de fibroina cargada de isoniazida eran esféricas. La eficacia de atrapamiento de las microesferas de formulación diferente de F1 a F5 estuvo en el rango de 53 a 68 %. F3 mostró un 68,47 % de eficiencia de atrapamiento y tras optimizar la formulación de liberación de fármacos fue de 93,56 %, a las 24 horas.
Conclusión: Esta investigación reveló una nueva formulación de base acuosa para las esferas de seda con forma controlable o la forma y el tamaño de la esfera. Las microesferas de seda cargadas de isoniazida pueden actuar como ideal formulación nano con estudios elaborados.
Palabras clave: fibroin de seda; nanoformulation; biodegradabilidad; isoniacida.
Introduction
Drug delivery research focused on micro and nano based delivery have made strong presence in healthcare industry. Microspheres based controlled delivery of antimycobacterial agents might be proficient through employing a variety of polymeric drug carriers. Even though knowledge among natural polymers is encouraging and extensive and may offer lots of benefits.1-5 Tuberculosis is most health crisis during the world or contaminate more than eight million persons every year. Oral therapy with the presently working anti tubercular drugs is extremely effectual and still related amid a number of important problems.6-9 Further than 80% of TB cases were of pulmonary TB unaided and soaring drug dose has essential to be managed since merely little slice of the entire dose reaches the lungs. Anti Tubercular drugs delivery system which could be delivered using the respiratory route alongside with canister evade the daily dosing since they can help out in: (i) Express drug delivery to the unhealthy person; (ii) The targeting of alveolar macrophages which have uses via the mycobacteria while a secure place for their extended survival; (iii) reduced systemic toxicity of the drugs; and (iv) improved patient compliance .Furthermore in distinction the oral route of administration and inhaled drugs were not subjected to first pass metabolism.It is not gratified for the reason that could not steadily reside in the lung.10-12 Silk fibroin show advanced mechanical properties and tunable degradation rates ranging from weeks to months in vivo due to control of excellent biocompatibility, crystallinity with low inflammatory and immunogenic response, and all aqueous material processing alternative to form films, gels, fibers, microspheres and sponges. For drug delivery, particularly protein drugs, silk materials exhibit controllable drug release kinetics and high encapsulation efficiency due to the crystalline beta-sheet formation.13-16 There are several techniques available for the preparation of drug loaded microspheres, such as evaporation or extraction methods, self-assembly, solvent displacement, emulsion-solvent phase separation, and spray drying. A new approach has preferred to fabricate silk microspheres through convenient sphere size, size and avoid with organic solvents along with a few extra insensitive circumstances during processing.17-21 Phase separation among polyvinyl alcohol and silk take place impulsively while the two polymer solutions are assorted and afterward casted into films. The influence of blending of PVA and silk on top of silk secondary arrangement changes also film mechanical and swelling properties was studied. The present study has to develop the phase separation of silk or PVA blends to produce silk spheres with controlled microspheres, size intended for efficacy in drug loading and release.22-26 Nearly numeral of recompenses to evolving sustained release formulation using silk fibroin for targeted drug delivery. PVA microspheres are most widely studied drug delivery systems for the controlled release of drugs that is vaccines, antihypertensive agents, antibiotics, peptide drugs, anti-cancer agents and Proteins.27-30 The focus of the current study was to prepare Silk fibroin microspheres by phase separation technique using polyvinyl alcohol (PVA).31-32 The inspirations of many particulate parameters stood investigated in relation to Microsphere performance and in-vitro drug release characteristics. The aim was to prepare Silk fibroin/PVA microspheres loaded with Isoniazid as drug delivery system which might enhance efficiency of Isoniazid aimed to local anti-tubercular activity and reduced the toxicity.
Materials and methods
The following materials were used: Isoniazid obtained from Balaji Drugs, Polyvinyl alcohol obtained from Fisher Scientific, Ethanol (Changshuyangyuan chemicals, China), Calcium chloride obtained from Avarice and Silk Cocoons collected from Himachal Pradesh. All reagents used were of analytical grade.
Silk fibroin purification
Silk fibroin aqueous stock solutions have been prepared from cocoons of Bombyxmoriouter.Silk cocoons was boiled for 30 min in 0.02 M sodium carbonate and after that rinsed meticulously with distilled water. The Silk Fibroin extracted was dissolved in ternary solvent mixture comprises of CaCl2: Ethanol: Water in the ratio of 1:2:8 respectively and boiled for 4 hour at 70 oC to form aqueous stock solution.33 Synthesis of Silk fibroin purification shown in figure 1
Preparation of Poly Vinyl alcohol Solution:
A 5% (wt/vol) PVA solution by adding 0.25 g of PVA to 4 ml of deionized water and heated at 60oC to dissolve the PVA.
Preparation of Isoniazid loaded Silk Fibroin Microspheres:
Microspheres were prepared using Phase separation method .The Microspheres were prepared by using different parts of Silk Aqueous Solution: Poly Vinyl alcohol Solution and Isoniazid drug were selected as independent variables. Initially Parts of Silk aqueous solution was kept in beaker under stirring at room temperature. Consequently, Poly Vinyl alcohol Solution was dissolved in Silk aqueous solution to obtain a known concentration according to the formulation design magnetic stirring at room temperature for 45 to 60 mins and sonicate the solution for 30 s at 25% amplitude. Pour the solution into a Petri dish and allow it to cover the bottommost of the dish consistently. Dry the Liquid solution over-night in a fume hood. The film be able to kept for a insufficient weeks. Seal and cover the dish with Parafilm and store. Peel off the film from the dish and place one film in a 50-ml conical flask or tube. Add 20 ml of deionized water to the flask or tube and shake for 30 min at room temperature to dissolve the film. Centrifuge at 13,000 r.p.m. for 25 min at 4oC and discard the supernatant. Resuspend the pellet in 5 ml of deionized water and used the suspension for Microspheres.34
Determination of Partition Coefficient:
An estimated partition coefficient was determined by shake flask method from the ratio (1:1, 1:2, 2:1) of solubility of isoniazid in n-octanol/chloroform to that in water. A stock solution of isoniazid was prepared in n-octanol/chloroform and water such that the maximum concentration of the test substance, in each phase, always remains below 1.2 absorbance during the experiment. The organic and aqueous phase was separate and aqueous phase was analyzed by UV spectrophotometric method at 262 nm and the partition coefficient was calculated as follows:35
Partition Coefficient; Log10 P n-octanol or chloroform/water =

Saturation concentration of drug in water
Saturation solubility study:
In this saturation solubility study, an excess quantity of Isoniazid was placed in the vial containing 10 ml and fill with deionized water. The vial was kept for 24 hours and the amount of the drug dissolved was analyzed after 24 hours using UV spectrophotometrically at 262 nm.36
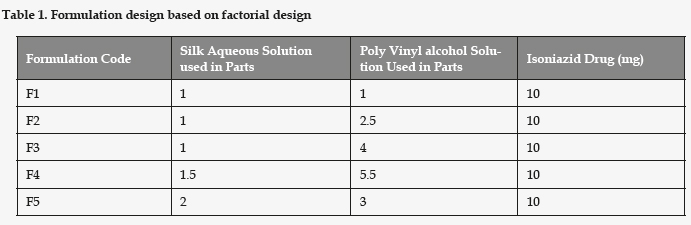
Formulation Design: Shown in Table 1
Evaluation of prepared Silk Fibroin of Microspheres.
Scanning electron microscopy
The samples were used for SEM solid as well liquid up to 50 mg or 0.5 ml liquid can be analyzed The morphologies of silk spheres were imaged using a SEM model No JEOL 5400 made by japan.Gold Ions Coating Is done 5-6 Mins. Images were taken with the installed software.
Entrapment efficiency (EE) of Microspheres
The entrapment efficiency of Microsphere was calculated using an indirect entrapment method. The prepared Microspheres suspension was centrifuged for 30 minutes at 40000 rpm. Then the supernatant is analyzed for the free drug content. The concentration of RIF in the supernatant was determined by UV-Visible spectrophotometry at 262 nm. The calculation was done to determine the amount of drug unentrapped in the supernatant. The amount of drug entrapped was calculated by subtracting amount of free unentrapped drug from the total amount of Isoniazid taken. The drug entrapment efficiency (EE) of microspheres was determined by using following formula:

Total quantity of drug
In-vitro drug release study using dialysis membrane
Preparation of dialysis membrane
The dialysis membrane (Mol wt 12000 KD) was soaked overnight in a buffer in order to open the pores of the membrane. After period of 24 hours dialysis membrane was taken out and was used for the in-vitro drug release.
In-vitro drug release of Isoniazid Loaded Microspheres
A dialysis membrane was used to monitor Isoniazid release from the Microspheres. Dialysis membrane with molecular weight cut off 12,000 KD was used to determine the release of isoniazid from Microspheres. For determining the in-vitro drug release the 7.4 phosphate buffer solution was prepared. The dialysis membrane was soaked overnight in a buffer in order to open the pores of the membrane. The dialysis membrane was taken out from the buffer and was tied at one end. The Prepared Isoniazid Microspheres was introduced into the membrane and the other end was also tied with thread so that the sample should remain in the membrane. The membrane was introduced in the buffer medium. At frequent time intervals 1 ml aliquot was withdrawn and after sufficient dilution was analyzed spectrophotometrically at 262 nm. The sample Withdrawn stayed replaced via an equal quantity of fresh 7.4 phosphate Buffer saline. The drug release profile is presented graphically.
Result and Discussion
UV Spectroscopy (λ max determination):
The Standard Solution of Isoniazid deionized water and pH 7.4 Phosphate buffer (10µg/ml) was Scanned (200-400) nm by using Double Beam UV-Visible Spectrophotometer (UV-1800) manufacturer by Shimadzu Corporation .The λ max was found to be 262 nm.
Melting point of Drug
Melting point was determined by thiele tube method and was found to be in the range of 170o - 172o C which is in accordance with reference value
Saturation solubility study
Isoniazid, showed a solubility of 210mg/ml, in deionized water, falling in the category of very soluble drug as per I.P. 2007
Partition Coefficient
A negative log P (-0.420) confirms the hydrophilic nature of isoniazid. The experimental solubility and partition coefficient values substantiate the suitable candidature of isoniazid for the presently proposed study on hydrophilic drugs.
Evaluation of prepared Silk Fibroin drug loaded Microspheres.
Scanning electron microscopy (SEM)
The morphologies of silk spheres were imaged using a SEM model No JEOL 5400 shown in Figure: 2
Entrapment efficiency (EE) of Isoniazid Loaded Microspheres.
The Entrapment Efficiency of Isoniazid loaded Microspheres were performed in triplicate different Formulation from F1 to F5 exposed in Table: 2 and Figure: 3
In-vitro drug release from Isoniazid Loaded Microspheres in 7.4 phosphate buffer:
In-vitro release study of drug from Microsphere solution in 7.4 Phosphate buffer obtained between Percentage (%) cumulative release and Time (hours). Figure: 4
Conclusion
Current experimental investigation was an attempt to develop a reconstituted microsphere of ant tubercular drug. Formulated silk microspheres were spherical, having narrow particle size distribution, glossy and dense surface, good entrapment efficiency and controlled drug release. The prepared microspheres could be used as a carrier for controlled release due to the biocompatibility and biodegradation of Silk Fibroin and long-term release of therapeutic proteins and peptides from Silk Fibroin matrix.
Discussion
Isoniazid loaded silk fibroin microsphere was prepared by using phase separation method. All batches of Microsphere were preliminarily evaluated. Results disclosed the formation of Silk Microspheres and its drug delivery applications. Scanning electron microscopy studies revealed that Isoniazid Loaded Silk Fibroin Microspheres were spherical. Entrapment Efficiency of Isoniazid loaded Microspheres of different Formulation from F1 to F5 was in range of 53 to 68 %. F3 showed 68.47 % Entrapment Efficiency and the optimized formulation drug release was 93.56 % at 24 hours. Stability Studies was Shows in 3 Months and no appreciable changes was noticed in drug release and Entrapment efficiency.
Acknowledgments
Author is also grateful to the CT institute of pharmaceutical sciences Shahpur, Jalandhar, Punjab for making available the research facilities used
References
1. Quenelle DC, Winchester GA, Staas JK, Barrow EL, Barrow WW. Treatment of tuberculosis using a combination of sustained-release rifampin-loaded microspheres and oral dosing with isoniazid. Antimicrobial Agents and Chemotherapy. 2001; 45:1637-1644. [ Links ]
2. Ain Q, Sharma S, Khuller GK. Role of poly (DL-lactideco-glycolide) in development of sustained oral delivery systems for antitubercular drug(s). Int J Pharmed. 2002;239:37-46. [ Links ]
3. Giovagnoli, Blasi P, Schoubben A, Rossi C, Ricci M. Preparation of large porous biodegradable microspheres by using a simple double-emulsion method for capreomycin sulfate pulmonary delivery. Int J Pharm. 2007; 333:103-111. [ Links ]
4. Cook RO, Pannu RK, Kellaway IW. Novel sustained release microspheres for pulmonary drug delivery. J Control Rel. 2005; 104:79-90. [ Links ]
5. Vasir JK, Tambwekar K, Garg S. Bio adhesive microspheres as a controlled drug delivery system. Int J Pharm 2003; 255:13-32. [ Links ]
6. Altman GH, Diaz F, Jakuba C, Calabro T, Horan, RL, Chen J, Lu H, Richmond J, Kaplan DL. Silk-based biomaterials. Biomaterials. 2003;24:401-416. [ Links ]
7. Eldridge JH, Hammond CJ, Meulbroek JA, Staas JK, Gilley RM, Tice TR. Controlled vaccine release in the gut-associated lymphoid tissues. I. Orally administered biodegradable microspheres target the Peyer's patches. J Cont Rel. 1990;11:205-214. [ Links ]
8. Cowsar DR, Tice TR, Gilley RM, English JP. Poly(lactide-coglycolide) microcapsules for controlled release of steroids. Methods Enzymol 1985;112:101-116. [ Links ]
9. Jacob E, Setterstrom JA, Bach DE, Heath JR, McNiesh LM, Cierny IG. Evaluation of biodegradable ampicillin anhydrate microcapsules for local treatment of experimental staphylococcal osteomyelitis. Clin Orthop Relat Res 1991; 267:237-244. [ Links ]
10. Mitchinson DA. The action of anti-tuberculosis drugs in short course chemotherapy. Int J Tuberc Lung Dis. 1985; 66:219-225. [ Links ]
11. Vedha Hari BN, Chitra KP, Bhimavarapu R, Karunakaran P, Muthukrishnan N, Samyuktha Rani B. Novel technologies: A weapon against tuberculosis, Indian J Pharmacol. 2010;42: 338-344. [ Links ]
12. Zhang J, Zhaoli D, Xu S, Zhang S. Synthesis and characterization of karaya gum/chitosan composite microspheres. Iran Polym J.2009;18:307-313. [ Links ]
13. Cao Z, Chen X, Yao J, Huang L, Shao Z. The preparation of regenerated silk fibroin microspheres. Soft Matter. 2007;3:910. [ Links ]
14. Zheng Z, Yi L, Mao-bin X. Silk fibroin-based nanoparticles for drug delivery. Int J Mol sci. 2015; 16:4880-4903. [ Links ]
15. Wang XQ, Yucel T, Lu Q, Hu X, Kaplan DL. Silk nanospheres and microspheres from silk/pva blend films for drug delivery. Biomaterials. 2010; 31:1025-1035. [ Links ]
16. Mitropoulos AN, Marelli B, Ghezzi CE, Applegate MB, Partlow BP, Kaplan DL, Omenetto FG. Transparent. Nanostructured Silk Fibroin Hydrogels with Tunable Mechanical Properties. 2015; 1 (10):964-970. [ Links ]
17. Kim HJ, Kim HS, Matsumoto A, Chin IJ, Jin HJ, Kaplan DL. Processing windows for forming silk fibroin biomaterials into a 3D porous matrix. Aust J Chem. 2005; 58:716-720. [ Links ]
18. Tanaka T, Tanigami T, Yamaura K. Phase separation structure in poly (vinyl alcohol)/silk fibroin blend films. Polym Int. 1998; 45:175-184. [ Links ]
19. Kamaly N, Yameen B, Wu J, Farokhzad OC. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. 2016; 116 (4):2602-2663. [ Links ]
20. Hino T, Shimabayashi S, Nakai A. Silk microspheres prepared by spray-drying of an aqueous system. Pharm Pharmacol Commun. 2000; 6:335-339. [ Links ]
21. Farago S, Lucconi G, Perteghella S, Vigani B, Tripodo G, Sorrenti M, et al. A dry powder formulation from silk fibroin microspheres as a topical auto-gelling device. Pharmaceutical Development and technology. 2015; 45: 1097-9867. [ Links ]
22. Philipp FS, Gregory TJ, Jelena RK, Yinan L, David LK. pH-Dependent anticancer drug release from silk nanoparticles. Adv Health Mater. 2013;2(12):1002-1011. [ Links ]
23. Wang X, Wenk E, Matsumoto A, Meinel L, Li C. Kaplan DL. Silk microspheres for encapsulation and controlled release. J Control Rel. 2007;117:360-370. [ Links ]
24. Wang Y, Kim HJ, Vunjak-Novakovic G, Kaplan DL. Stem cell-based tissue engineering with silk biomaterials. Biomaterials. 2006; 27:6064-6082. [ Links ]
25. Winkler S, Wilson D, Kaplan DL. Controlling beta-sheet assembly in genetically engineered silk by enzymatic phosphorylation/ dephosphorylation. Bio chem. 2000; 39:12739-12746. [ Links ]
26. Wang X, Wenk E, Matsumoto A, Meinel L, Li C, Kaplan DL.Silk microspheres for encapsulation and controlled release. J Con Rels. 2007; 117:360-370. [ Links ]
27. Dai L, Li J, Yamada E. Effect of glycerin on structure transition of PVA/SF blends. J Appl Polym Sci. 2002; 86:2342-2347. [ Links ]
28. Xiaoli Z, Keyong T, Xuejing Z. Electrospinning and Crosslinking of COL/PVA Nanofiber-microsphere Containing Salicylic Acid for Drug Delivery. J Bio Eng. 2016; 13:143-149. [ Links ]
29. Segi N, Yotsuyanagi T and Ikeda K. Interaction of calcium-induced alginate gel beads with propranolol. Chem. Pharm. Bull. 1989; 37 (11): 3092-3095. [ Links ]
30. Dinesh Kaushik, Satish Sardana and DinaNath Mishra. In-vitro characterization and cytotoxicity analysis of 5-Fluorouracil loaded chitosan microspheres for targeting colon cancer. Ind J Pharm Educ Res. 2010; 44 (3):274-282. [ Links ]
31. Rosca ID, Watari F, Uo M. Microparticle formation and its mechanism in single and double emulsion solvent evaporation. J Control Release. 2004; 99:271-28. [ Links ]
32. Qiang L, Haifeng L, Yubo F. Preparation of silk fibroin carriers for controlled release. Wiley periodicals, inc. 2015;8:1-9. [ Links ]
33. Lammel A, Hu X, Park SH, Kaplan DL, Scheibel T. Controlling silk fibroin particle features for drug delivery. Biomaterials. 2010; 31(16): 4583-4591. [ Links ]
34. Rockwood DN, Preda RC, Yücel T, Wang X, Lovett ML, Kaplan DL. Materials fabrication from Bombyx mori silk fibroin. 2011;6(10):1612-1631. [ Links ]
35. Rodrigues C, Gameiro P, Reis S, Lima JL, Castro B. Spectrophotometric determination of drug partition coefficients in dimiristoyl- L-a-phosphatidylcholine/water: a comparative study using phase separation and liposome suspensions, Anal Chim Acta. 2001; 428:103-109. [ Links ]
36. Miranda L, Cheney, Shan N, Elisabeth R, Hanna M, Wojtas L, Michael J, Zaworotko, Sava V, Juan R, Sanchez-Ramos. Effects of Crystal Form on Solubility and Pharmacokinetics: A Crystal Engineering Case Study of Lamotrigine. Cryst Growth & Des. 2010;10: 394-405. [ Links ]
![]() Correspondence:
Correspondence:
Narinder Singh
+91 9464394755
pharmacist.narinder@gmail.com
Received: 15-05-2016
Accepted: 11-09-2016