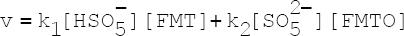INTRODUCTION
famotidine(FMT),3-[2-(diaminomethyleneamino)thiazol-4ylmethylthio]-N-sulfamoylpropionamidine, is a histamine H2-receptor antagonist (H2-RA) which competitively inhibits the action of histamine on the H2-receptors of parietal cells and thereby reduces the gastric acid secretion under daytime and nocturnal basal conditions. It is easily oxidized and the metabolytes are S-oxides which can be impurities in the medical preparation. The metabolite has no pharmacological activity on gastric acid secretion1. It is produced in the form of powder substance and tablets containing 20 or 40 mg of API and other pharmaceutical formulations.
The British Pharmacopoeia2 recommends a potentiometric nonaqueous method for the determination of FMT using perchloric acid as the titrant, while the USP3 recommends a similar approach for the determination of FMT in its bulk form, and an HPLC method using a mixture of acetate buffer of pH 6: acetonitrile (93:7) as a mobile phase with UV detection at 275 nm.
An extensive literature survey revealed that FMT has been estimated in pharmaceuticals by spectrophotometry. Spectrophotometric techniques provided practical (less-time-consuming, simple, and more convenient) and significant economic advantages over other methods; therefore, they are a frequent choice for pharmaceutical analyses 4 5-6. HPLC methods generally required complex and expensive equipment, provision for use and disposal of solvents, tedious sample preparation procedure, and personal skills in chromatographic techniques 7 8 9 10-11. Other methods used for FMT quantitative determination belong to capillary electrophoresis 12 and electrochemical methods 13 14 15 16-17. Some of these methods have enough sensitivity to determine lower concentration of the drug, however, it is always required to develop simple, fast, inexpensive analytical methods that can be readily adopted for routine analysis at relatively low-cost to the different requirements of analytical problems.
Titrimetry is still considered to be very convenient and economical techniques for routine analysis of the drug in pharmaceutical formulations. The FMT content has earlier been determined titrimetrically based on the reaction of drug with chloramine-T18.
The purpose of the present work is to study kinetics of Famotidine S-oxidation products formation by means of Caro’s acid and to develop and validate iodometric procedure for determination of FMT in pure substance and tablet formulation.
MATERIALS AND METHODS
All materials were of the analytical reagent grade, and the solutions were prepared with twice-distilled water.
Famotidine pure substance, Pharmaceutical grade (ac No. FMC/1508003, FM-1507002V 24/08/2015, Nakoda Chemicals Ltd,product-E-P) was used as received. Famotidine preparation, tablets, 20 mg, produced by PJSC «Kyivmedpreparat», Ukraine was used for the research (Certificate No.0010713 24/04/2013). The oxidant was KHSO5, potassium caroate in the form of a triple potassium salt of Caro’s acid, 2KHSO5 × KHSO4 × K2SO4 (Acros Organics). The choice of the reagent was determined by its rather high oxidative capacity, E0 = 1.84 V19, easy availability, and satisfactory solubility in water, and also by sufficiently high stability in the use and storage.
Preparation of standard solutions
Preparation of 0.02 M potassium caroate solution. 0.615 g of 2KHSO5•KHSO4•K2SO4, was dissolved in twice-distilled water (100 mL). The concentration of potassium caroate was controlled by iodometric titration.
Preparation of 0.005 M Famotidine standard solution. 0.17 g (precise weight) of FMT substance was dissolved 5 mL of 0.1 M HCl solution and after the complete dissolution the volume was brought to the mark with double-distilled water (100 mL).
Preparation of 0.02 M sodium thiosulfate solution. A 0.1 mol L-1 solution of sodium thiosulfate was prepared from the standard titre fixanal. A 2/10 dilution was made to obtain required concentration.
Preparation of 5 % potassium iodine solution. 5.0 g of potassium iodine was weighted and in 100 mL of distilled water.
Preparation of 0.1 M sulfuric acid solution. The solution was prepared from the standard titre fixanal in a 500 mL volumetric flask.
Preparation of buffer solutions
For pH= 2.30: Dissolve 20.1467 g of C6H8O7 H2O and 1.4604 g of Na2HPO4 2H20 in 1000 mL of distilled water.
For pH= 3.60: Dissolve 14.2434 g of C6H8O7 H2O and 11.4696 g of Na2HPO4 2H20 in 1000 mL of distilled water.
For pH= 5.00: Dissolve 10.1889 g of C6H8O7 H2O and 18.3443 g of Na2HPO4 2H20 in 1000 mL of distilled water.
For pH= 7.00: Dissolve 3.7079 g of C6H8O7 H2O and 29.333 g of Na2HPO4 2H20 in 1000 mL of distilled water.
For pH= 8.40: Add 8.00 mL of 0.1 mol L-1 HCl solution to 250 mL of 0.2 mol L-1 Na2HPO4 solution.
Apparatus
pH meter. I-160M Gomel, the Republic of Belarus ESKL-43-07 with glass electrode as indicator was used for pH measurements.
Titration. The titrant volume was measured using a 10 mL microburette with the accuracy of ±0.01 mL.
Voltammetric measurements were carried out on the digital device20 equipped with personal computer and temperature-controlled three-electrode cell, volume 10 mL. An indicator dropping mercury electrode (DME), a saturated calomel reference electrode and platinum wire auxiliary electrode were used. The dropping mercury electrode employed had the following characteristics: m=5.94 · 10-4 g s-1; tk=10 s in 0.2 M universal buffer solution with open circuit.
IR spectrum of S-oxide prodrug of FMT exhibits characteristic peak at 3401.8 NH stretching 1330.5 cm-1 asymmetric SO2 stretching vibration, 1251.7 cm-1 aliphatic CN stretching , 667.2 cm-1 S-N stretching vibration.
All the procedures have been performed at room temperature (T=293 K).
Procedure
Studying of Famotidine S-oxidation kinetics. 10,00 mL portion of 0.02 M KHSO5 solution was transferred into 100 mL volumetric flask, 10.00 mL of 0.02 M of NaOH solution and 10 mL of standard FMT solution was added and brought to the mark with corresponding buffer solution, stirred vigorously and left for 1 min. After the addition of FMT solution a stopwatch was switched on. During the first 40 min every 5 min such a procedure has been performed: 10.00 mL aliquot of the mixture obtained was transferred using the pipette into titration flask, 1 mL of 0.1 M sulfuric acid solution and 1 mL of 5 % potassium iodine solution were added. The isolated iodine was titrated by 0.02 M solution of sodium thiosulfate (V, mL).
The control experiment was carried out in the same conditions paralleled (without FMT with the same amount of KHSO5 0.02 M solution (V0, mL)). Each one mL of 0.01 M solution of sodium thiosulfate is equivalent to 0.001350 g of FMT (C8H15N7O2S3) (CAS number 0076824-35-6) (content of FMT is in the limits 98.5-101.5%).
Famotidine pure substance quantitative determination procedure. 10,00 mL portion of 0.02 M KHSO5 solution was transferred into 100 mL volumetric flask, 10.00 mL of 0.02 M of NaOH solution and 5,00 mL; 10,00 mL or 20,00 mL portion of standard FMT solution was added and brought to the mark with pH=7.0 buffer solution, stirred vigorously and left for 1 min. After the addition of FMT solution a stopwatch was switched on. The demand of oxidizing reagent is quantitative and stoichiometric. The time of interaction finishes in 20 min. Further experiment as for Studying of Famotidine S-oxide kinetics.
Famotidine tablet formulation quantitative determination procedure. 15 powdered tablets of FMT containing 20 mg of the active substance have been dissolved in 100 mL volumetric flask (see FMT standard solution preparation). The solution obtained has been filtrated through filter paper. Further experiment as for Famotidine pure substance quantitative determination procedure. The robustness of the procedure has been checked through the performance of the experiment in two different days.
Method validation
The method was validated according to the guidelines of the International Conference on Harmonization21.
The Precision and Accuracy on these procedures were investigated with respect to repeatability and determined by performing five repeated analysis of the samples on the same day, under the same experimental conditions.
LOD and LOQ were calculated from regression equation as 3.3 S0/b and 10 S0/b respectively, where S0 and b are standard deviation slope of the calibration curve.
Method comparison. Results obtained in this study were compared to those given in the quality certificates for the pure substance and tablets, i.e. HPLC method.
RESULTS AND DISCUSSION
Kinetic studies were carried out in water medium under second-order conditions with potassium caroate at the temperature 293 K. The reaction was followed by estimating the unreacted Caro’s acid as a function of time using the iodometric method. The isolated iodine was titrated against standard sodium thiosulfate solution using starch as indicator.
Metamorphosis of the kinetic curves 1/c vs t are given on the Figure 1. The linear dependence reveals the second order reaction (Fig. 1). As it is seen from the plot in the pH value interval 2.3-8.4 the rate of chemical reaction increases.
Peroxoacidic titration of standard solutions was carried out to determine the stoichiometry of the reaction.
Stoichiometry of the reaction at the pH 7.0-8.4. From the kinetic curves it is clearly seen that 1 mol of Famotidine is oxidized by 2 mols of KHSO5. The quantitative formation of Sulfone is observed during the time that doesn’t exceed 30 min (FMT Sulfone formation from FMT S-oxide).
The formation of Famotidine S-oxide is immediate (during the first minute). The observed rate constants (kobs) show the formation of FMT Sulfone. The reaction rate is fast during the first 20 min, but later the reaction slows down (Fig. 2..).
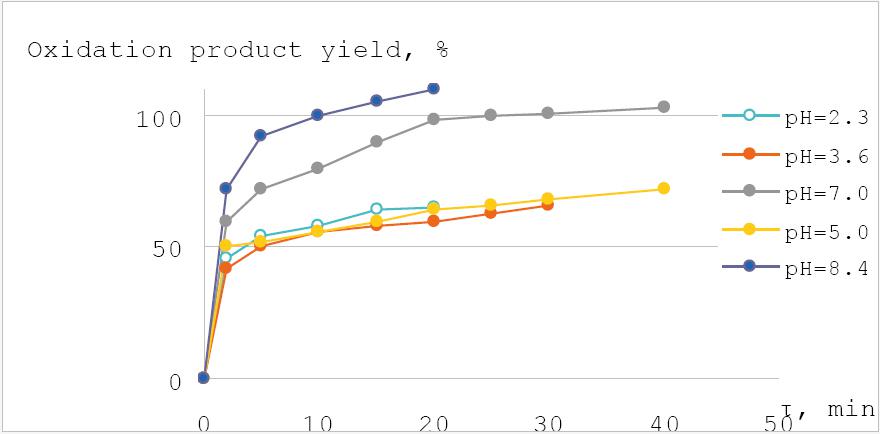
Fig. 2 Dependence of oxidation product yield (%) on time (min). c(FMT) = 5×10-4 M; c(KHSO5) = 2×10-4 M
FMT is polarographically nonactive, but it is oxidized by the potassium caroate and is reduced on the mercury drop electrode. The FMT S-oxide is formed in the acidic medium (pH=3-7) at room temperature. Its corresponding peak equals E= -0.6 ÷ -0.8 V. The FMT Sulfone is formed in the basic medium (pH=7-9) and its corresponding peak is in the limit E= -1.4 ÷ 1.5 V. These values can be used as identifications to prove the formation of the corresponding product.
The product was isolated and characterized by IR-spectrum. The shift in S-N stretch occurs in S-oxide prodrug when compared to that of FMT indicates the formation of a S-oxide22.
The oxidation depth is controlled to a greater extent by the pH of the reaction mixture. Potassium caroate is present in the form of HSO5– and SO52– ions in solution and they are weak and strong nucelophiles respectively. It is suggested that the reaction proceeds through an nucleophilic attack of the oxidant (HSO5–) on the electrophilic site sulfur formed in the first step of the FMT S-oxide reaction (Fig. 3,a) by mince a mechanism involving displacement of terminal oxygen of the peroxide group. A cyclic intermediate undergoes intramolecular rearrangement to give FMT Sulfone (Fig. 3,b) as the product23.
A hypothetic mechanism scheme based on these observations is proposed on Figure 3:
The corresponding kinetic equation is following:
This, in particular, points to a linear dependence of the observed reaction rate constant on the molar fraction of the dianion of the Caro’s acid.
The fact that the maximum rate (second order) falls at pH value equal to pK2 value (about 9.4) of Caro’s acid24 explains that at this pH value the highest rate of spontaneous decomposition of Caro’s acid (attack of monoanion on dianion) is observed. That is why such pH of the medium should be avoided. Indeed, the overexcess of the oxidation reagent is observed at the pH=8.4. In the research work of Pylypchuk and others have studied the stability of diluted solution of Caro’s acid depending on the temperature and pH values. In the conclusion optimal pH 3-4 have been recommended as the most stable at room temperature25. So, taking into account all the mentioned above and the results obtained during the experiment (Fig.4) the optimal pH for FMT Sulfone formation is 7.0. At this pH value sulfone derivates are formed with satisfactory rate (20 min) plus uncontrolled decomposition of the oxidizing agent in an auxiliary reaction.

Fig. 4 The dependence of the observed reaction rate constant on pH (1) and on the molar fraction of the dianion of the Caro’s acid (2) (r=0.990).c(FMT) = 5×10-4 M; c(KHSO5) = 2×10-4 M
The proposed method was validated statistically for Famotidine pure substance and medical preparation. The iodometric back titration method equation was used for the calculations. The results are shown in the Table 1.
Table. 1. Results of Famotidine pure substance accuracy and precision calculation
| Added, M 103 | Found, M 103 | Mean, M 103 | Recovery, % | RSD, % | δ*, % |
|---|---|---|---|---|---|
| 2.50 | 2.45 | 2.48 | 99.20 | 1.70 | 0.80 |
| 2.50 | |||||
| 2.47 | |||||
| 2.55 | |||||
| 2.45 | |||||
| 5.00 | 5.10 | 5.02 | 100.40 | 1.53 | 0.40 |
| 4.96 | |||||
| 4.96 | |||||
| 5.15 | |||||
| 5.10 | |||||
| 10.00 | 6.97 | 10.05 | 100.50 | 1.09 | 0.50 |
| 6.97 | |||||
| 7.10 | |||||
| 7.19 | |||||
| 6.97 |
*Calculated using the certificate data obtained by the HPLC method
The method is linear in a wide range: 1-10 mg mL-1 with the equation Y = (0.9954±0.13)X, R=0.999. The calculated LOD = 0.01 mg mL-1 and LOQ = 0.03 mg mL-1 show high sensitivity of the procedure proposed.
The procedure was approbated for the tablet form. The results have been calculated by the method of standards corrected by the average tablet mass. The data obtained are shown in the Table 2.
Table. 2. Results of Famotidine tablets accuracy, precision and robustness calculation
| Level | FMT, tablet formulations, mg | |||||
|---|---|---|---|---|---|---|
| Day 1 | Day 2 | |||||
| 41.6 | 20.8 | 10.4 | 41.6 | 20.8 | 10.4 | |
| 1 | 39.9 | 21.1 | 10.9 | 41.7 | 20.9 | 10.2 |
| 2 | 39.9 | 20.9 | 10.9 | 41.7 | 20.4 | 10.2 |
| 3 | 41.7 | 21.3 | 10.4 | 41.7 | 21.1 | 10.4 |
| 4 | 42.6 | 20.4 | 10.4 | 42.6 | 21.3 | 10.4 |
| 5 | 42.6 | 21.1 | 10.2 | 40.8 | 21.3 | 10.9 |
| Mean, mg | 42.06 | 20.96 | 10.56 | 41.70 | 21.00 | 10.43 |
| RSD, % | 1.17 | 1.64 | 2.87 | 1.53 | 1.78 | 2.66 |
| d*, % | 1.11 | 0.77 | 1.54 | 0.24 | 0.96 | 0.29 |
*Calculated using the certificate data obtained by the HPLC method
CONCLUSIONS
The kinetics of Famotidine S-oxide and Sulfone formation by means of potassium caroate have been studied. Optimal conditions for the Famotidine Sulfone formation have been proposed (pH=7.0, t=20 min).
Titrimetric method is described for the determination of Famotidine in pure substance and tablet formulations using potassium caroate as oxidimetric agent. The procedure proposed is linear for pure substance in the interval 1-10 mg mL-1 and r=0.999 with the recovery percent ranged from 99.2 to 100.5%, RSD from 1.09 to 1.70 % and LOQ = 0.03 mg mL-1 for pure substance.
The validation has been performed for tablet formulations with good results obtained. RSD for tablet formulations has been in the limits from 1.17-2.87 % (d = -0.24÷-1.54 %).
The proposed procedure is demonstrated to be simple and cost-effective compared to many reported methods including the official ones.