INTRODUCTION
Preservatives are substances that are commonly added to various pharmaceutical preparations and food products in order to prolong their shelf life1,2.
The addition of preservatives to such products, especially to those that have higher water content, is essential for avoiding alteration and degradation by microorganisms during storage. They are used in sterile preparations such as eye drops and multi-dose injections to maintain sterility during application3. However these preservatives may be harmful to consumer due to their tendency to induce allergic contact. Hence the simultaneous determination of these preservatives in commercial pharmaceutical products is particularly important both for the quality assurance and consumer safety.
Several chemical derivatives of the quaternary ammonium ion have been used as preservatives. In each instant, the added ingredient must be harmless in the amount used; does not exceed the minimum quantity required to provide its intended effect, its presence should not impair the bioavailability, the therapeutic efficacy or safety of the official preparation, and should not interfere with analysis and tests prescribed for determination of compliance with the pharmacopeias standards.
The most frequently used individuals are benzalkonium chlorides (BAC). BAC is a mixture of alkylbenzyldimethyl ammonium chlorides with the general formula [C6 H5 CH2 N(CH3)2 R]+ Cl-,where R = CnH2n+1, n= 8,10,14,18 4.
Literature survey revealed that few analytical methods have been reported for the estimation of BAC in pharmaceutical preparations by extraction spectrophotometry2, capillary electrophoresis5, and high performance liquid chromatography6-7. These methods were related with some major drawbacks such as having inadequate sensitivity, being time-consuming, tedious, and dedicated to sophisticated and requiring expensive instruments.
There are several reviews for the analysis of preservatives in food, wood polymers, biological samples, and cosmetics. However, the literature is poor in terms of a comprehensive review in the analysis of preservatives collectively in pharmaceutical products, except of the review published in Chinese language by Ho and Chen8.
Nowadays, enzymatic biosensors are used for determination of cationic surfactants. Most often their application is based on the inhibition of the cholinesterase (ChE). The mechanism of analytical reaction and sensitivity of this method are the same as, or similar to, those in the human body. The conventional enzymatic method is based on the determination of the rate of ChE hydrolytic decomposition of the neurotransmitter (substrate) acetylcholine (ACh) to the choline or acetic acid. Due to the intensity of the color in time increasing, it was used as an analytical signal.
An enzyme conductometric biosensor based on acetylcholinesterase inhibition for the determination of BAC in aqueous solutions is described 9-10. It is based on the registration of conductometric transducer of conductivity solution of the enzymatic reaction product - Acetic acid ions. Several variants of inhibitor determination were examined. The linear range for the determination of BAC was observed from 0.75 to 20 mgL−1.The sensitivity at determination of BAC was 0.35 mgL−1, at the determination of BAC (8.75 mgL−1) relativ standard deviation (RSD) was shown 11%. However, the time stage of washing influences on the analytical signal (conductivity of solution), and the time of whole procedure can reach the 40-70 min9,10.
The kinetic photometric method offers an easy, less time consuming, sensitive analysis, using simple and available reagents, which are able to be used for routine determinations of drug substances, therefore kinetic spectrophotometric analysis is one of the major interests of analytical pharmacy.
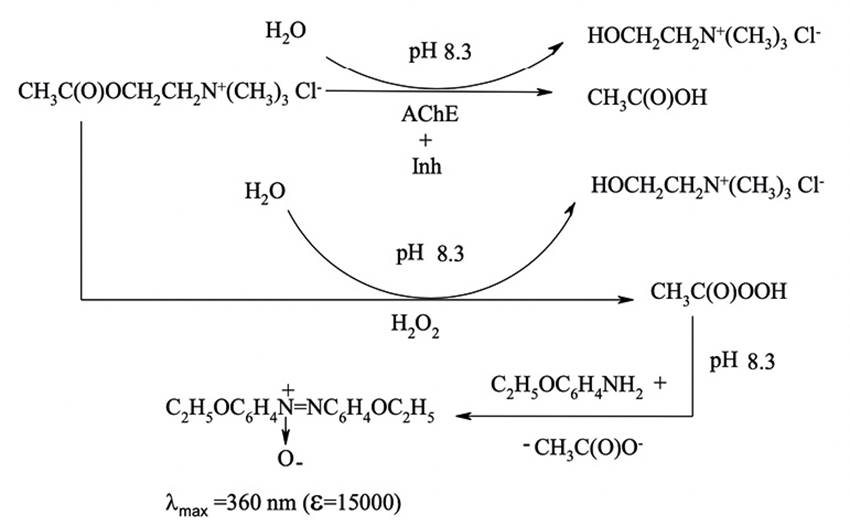
This work represents the first attempt at assaying of BAC in pharmaceutical preparation “Optrex» by the use of the novel enzymatic kinetic photometric method. The reaction rate is detected by the unreacted ACh, which is non- hydrolysed in the reaction with H2O2. As the result, 4,4’- azoxyphenetole was formed. (Processes underlying the analytical determination are shown in Figure 1) The measurement of the rate of changing of light absorption vs time (tg α, min-1) can be used for quantitative determination of BAC. The dependence of inhibition degree on the inhibitor concentration was used for plotting of the calibration curve. Its linearity allows to assay the BAC by the standard method and/or standard additions procedures. The proposed method is simple, accurate and sensitive.
EXPERIMENTAL
Materials and methods:
Benzalconium chloride (50.0% in water by certificate). Description composition: BAC 50% Ph.Eur., USP/NF consists of benzyl (dodecyl) dimethyl ammonium chloride (approx. 65%) and benzyl (tetradecyl) dimethyl ammonium chloride (approx. 35%). CAS No.: 8001-54-5 EINECS. Alkyl dimethyl benzyl ammonium chloride. Chain length: 60-70% w/w C12, 30-40% w/w C14 and max. 5% w/w C16. Activity Mw =352.5 g/mol, produced by Akzo Nobele, Surface Chemistry AS, Sweden.
p-phenetidine (4-ethoxyaniline - 98%), (chem. pur.); СAS -156-43-4; A0281408 series, produced by Sigma - Aldrich New Jersey, USA; p-phenetidine hydrochloride (Ph), extracted from the base by hydrogen chloride precipitation in the chloroform solution.
Acetylcholine Chloride (Pharm Grade) - 0.2 g per amp/5 mL, produced by “Vector” - State Science Center of virology and biotechnology in Russian Federation” (Russia).
Pharmaceutical preparation “Optrex” is a scientifically prepared eye lotion for care of eyes. It contains: distilled witch hazel B.P.C.13.0% (v/v): preserved with benzalkonium chloride 0.005 % (w/v) in a solution buffered with borax and boric acid (produced by Reckitt Benckiser Healthcare International Ltd).
Sodium phosphate dibasic, Na2HPO4∙12H2O (puriss.), CAS -7558-79-4, produced by «ReaChem», Kharkiv, Ukraine.
Dry protein drug of cholinesterase from horse serum - 80 mg / fL (VI class), 22 AU/mg, produced by SMU “Biomed”, Russia.
Remark: The catalytic activity of 1 unit (U) has such amount of the given enzyme preparation which coverts 1 µmole of the given substrate in 1 min at the given reaction conditions.
“Stabilized hydrogen peroxide 30-40%”, (puriss.), (LLC “Inter - Synthes”, Boryslav, Ukraine); the content of hydrogen peroxide was determined by SPU according to the monograph “High-test hydrogen peroxide solution 27.5-31.0%
High purity double distilled water was used throughout.
The absorbance measurements were performed on colorimeter (CFC-2) (Zagorsky Optical & Mechanical plant, Russia) using quartz cells of 2- cm path length.
The pH measurements were performed with a combined glass electrode (SP20B) together with auxiliary chloride silver electrode of EAL-1М3.1 type standard with potassium chloride.
A standard stock solution was prepared using double distilled water: 0.09910g of BAC standard solution (50.0%) was quantitative transferred in a 500 mL volumetric flask and it was diluted by water. 1.00 mL of this solution was transferred into a 10 mL volumetric flask and was diluted to mark.
Preparation of 0.2 M Phosphate buffer solution (pH 8.35)
35.75 g disodium hydrogen phosphate dodecahydrate (grade «p.a.»), crystallized (Na2HPO4·12H2O) was dissolved in 500 mL flask using double-distilled water. 19 mL of 0.1 M solution of hydrochloric acid solution was added. pH of the final solution was controlled potentiometrically.
Preparation of 10% hydrogen peroxide solution.
The solution was prepared by the appropriate high-test hydrogen peroxide dilution with double-distilled water. The content of hydrogen peroxide in the working 10% solution was determined by permanganatometric method.
Preparation of 1% p-phenetidine hydrochloride solution.
p-Phenetidine hydrochloride (Ph), was extracted from the base by hydrogen chloride precipitation in the chloroform solution. 1.00 g of p-phenetidine hydrochloride was dissolved in 80 mL of double-distilled water in 100 mL volumetric flask and after the dissolution brought to the mark.
Preparation of acetylcholine chloride solution (ACh).
The ampoule’s content 0.2 g of pharmacopoeia drug ACh was dissolved in 200 mL of double-distilled water. For that end, an ampoule was opened, 4.0 mL of water was pipetted, and shaked until acetylcholine was completely dissolved. Then the Acetylcholine solution was transferred into 200 mL volumetric flask and the volume was brought to the mark with double-distilled water.
General recommended procedure
The first part: 10.0 mL portion of 0.2 M phosphate buffer solution (pH = 8.3) was transferred into 20 mL graduated test tube with ground plug, 1.0 mL of 1% acetylcholine solution was added and then 2.0 mL of 10% hydrogen peroxide solution was added and the stopwatch was switched on. After that the solution was shaked thoroughly and was thermostated for 10 min. Then 1.0 mL of 1% p-phenetidine solution was added and brought to the mark with distilled water in a 20 mL volumetric flask. The stopwatch was switched on and every minute each solution was scanned photometrically for 15 min on photoelectric colorimeter, color filter No. 2 and 1.0 cm cuvette were used. The rate of reaction was determined as a slope of the kinetic curve A vs time [(ACh + H2O2) + p-Ph] (tg α, min-1).
The second part: 10.0 mL portion of 0.2 M phosphate buffer solution (pH = 8.3) was transferred into 20 mL graduated test tube with ground plug. After that accurate 2.0 ml portion of cholinesterase was added, then 2.0 mL of 10% hydrogen peroxide solution was added while stirring, shaked up thoroughly and kept for 10 min in a thermostat. Then 1.0 mL of 1% p-phenetidine solution was added and brought to the mark with distilled water. The stopwatch was switched on and every minute the solution was scanned photometrically for 15 min on photoelectric colorimeter, color filter No. 2 and 1.0 cm cuvette were used. Buffered solution with double - distilled water as reference solution was used. The rate of reaction was determined as a slope of the kinetic curve A vs time, (ChE) + ACh)t + H2O2 + p-Ph, (min-1) switched on a stopwatch, and thermostated for 10 min, [(ChE) + ACh) + H2O2 + p-Ph]tgamin (min-1).
The third part: 10.0 mL portion of 0.2 M of phosphate buffer solution (pH = 8.3) was transferred into 20 mL graduated test tube with ground plug. The accurate volumes of test solution of BAC were added into a standard flask. 2.0 mL of cholinesterase was added while stirring, the stopwatch was switched on, every solution was shaked up thoroughly and thermostated for 10 min, then quickly 1.0 mL of 1% acetylcholine solution was added and the stopwatch was switched on, hacked thoroughly and thermostated for 10 min again, then 2.0 mL of 10% Hydrogen peroxide solution was added, kept for 10 min in thermostat and after 1.0 mL of 1% p-phenetidine solution was added and brought to the mark with distilled water. The stopwatch was switched on and every minute the solution was scanned photometrically for 15 min on photoelectric colorimeter, color filter No. 2 and 1.0 cm cuvette were used. Every time before the experiment the test tube content was shaked and plugged thoroughly. Buffered solution with double - distilled water as reference solution was used. The rate of the reaction was determined as a slope of the kinetic curve A vs time)
[( ChE + Inh) + Ach] +H2O2 + p-Ph], tgaci (min-1).
Calibration graph procedure
The performances of the proposed method were verified on samples containing from 1.00 mL to 5.00 mL of BAC solution (WSS) according to the General procedure.
The degree of inhibition of the enzymatic hydrolysis of acetylcholine U, %, in the presence of BAC was calculated using the formula:
where,
tgaci - slope of the kinetic curve A vs time for a procedure [(ChE + Inh) + Ach] +H2O2 + p-Ph, tgaci (min-1);
tgamin- slope of the kinetic curve A vs time for a procedure [(ChE + ACh) + H2O2 + p-Ph], tgamin (min-1) ;
tgaVmax- slope of the kinetic curve A vs time for a procedure [(ACh + H2O2) + p-Ph], tgaVmax (min-1).
RESULTS AND DISCUSSION
Preliminary experiments showed that the parameters that can influence the performance of the proposed method. They were studied to reach the optimum of working and reagent concentrations11. Once the optimum working conditions were established, we have evaluated enzymatic, kinetic photometric method with respect to linearity, LOD, accuracy, precision.
Kinetic curves (Figure 2) of analytic indication reaction of p-phenetidine oxidation by hydrogen peroxide in the presence of the system: [(ChE + Inh) + Ach] +H2O2 + p-Ph, tgaci , [(ChE + ACh) + H2O2 + p-Ph], ACh+(ChE+BAC), [(ACh + H2O2) + p-Ph] were linear, for the first 15 minutes.
This enables the use for assessing of the reaction rate the slope angle tangent (angular coefficient of slope) of the derived kinetic lines, built in the coordinates optical density (A) - time (t, min) min-1 as the value of the analytical signal, corresponding to a certain content of an inhibitor in a sample.

Figure 2: Kinetic curves of couple oxidation of p-phenetidine by hydrogen peroxide in the system: 1 - ACh+ChE, 2- 6 -ACh+(ChE+BAC) , 7 - AСh. w(ACh) = 0.1%; [ChE] = 0.25 U; c(BAC), 10-6 M: 2-1.4, 3- 2.8, 4-3.4, 5 -5.6 , 6 - 7.0.
The calibration graph was constructed using the values obtained from five replicate samples of the same BAC content. The linear regression equation was as follows: U (%) =0.7434c·107+5.54 (where «c» is the BAC concentration expressed in mol L- 1; b = (0.74±0.04)·107; a = 5.5±2.0).
The calibration curve was linear in the concentration range of (1.4- 8.4)·10-6 M of BAC with a correlation coefficient of 0.999. The limit of determination was calculated as 20% degree of ChE inhibition and was 1.9·10-6 M.
In order to estimate the accuracy and precision of the proposed method, standard solutions of 2.8·10-6; 4.2·10-6; 5.8·10-6 and 7.0·10-6 M were analyzed according to the recommended procedure. For this purpose, five replicate determinations of each concentration were prepared. In graduated test tubes with ground plug 8.0 mL of 0.2 M phosphate buffer, and respectively, 2.00 mL, 3.00 mL, 4.00 mL and 5.00 mL of BAC solution (WSS) were added gradually in each one and analyzed according to the above procedure of calibration curve. The results of BAC assay in standard solution are presented in Table 1. As can be seen in Table 1, the percent recovery ranged 100.71 % to 101.07% (texp<ttabl), while the relative standard deviations ranged from 0.71 % to 2.95 %.
Table 1: Precision and Accuracy of the proposed Method
| BAC taken | BAC found* | RSD % | Recovery % | t exp (t table =2.78) |
|---|---|---|---|---|
| c ·10 6 , mol L -1 | ||||
| 2.80 | 2.83±0.10 | 2.95 | 101.1 | 0.82 |
| 4.20 | 4.22±0.08 | 1.57 | 100.5 | 0.68 |
| 5.60 | 5.65±0.06 | 0.86 | 100.9 | 2.22 |
| 7.00 | 7.05±0.60 | 0.71 | 100.7 | 2.24 |
* N = 5; P =0.95 %. RSD = Relative standard deviation; BAC - benzalkonium chloride.
The proposed method was applied to the analysis in the eye drops formulation “Optrex.
Recommended procedure for the analyses of eye drops “Optrex” solution
1.00 mL of the eye drops “Optrex” solution was transferred into 5 mL volumetric flask and brought to the mark with double distilled water.
Known volumes of the sample solution (1.00 mL) were analyzed by the proposed kinetic-photometric method (according to general procedure) (Table 2).
Accuracy and reliability of the proposed method were further ascertained by performing recovery experiments. To a fixed amount of the drug in formulation (pre-analysed), pure BAC at three different levels of concentration was added, and the total content of BAC was determined by the proposed method.
10 mL of phosphate buffer 0.2 mol L-1 solution were transferred into each graduated test tube with ground plug gradually, 1.00 mL of eye drops “Optrex” sample solution and 2.00 mL, 3.00 mL, 5.00 mL of Benzalkonium chloride solution (WSS) were added respectively. After see General procedure. Each test was repeated three times. Linear calibration graph was U (%) =0.747c·107+5.54 and regression coefficient of 0.9995 is obtained. The results show that recoveries were in the range 100.79 - 101.19 % indicating that commonly active pharmaceutical ingredient (API) did not interfere the kinetic photometric determination of the BAC. Thus, the proposed method shows the accuracy and reliability results: (texp<ttable) and RSD < 2.8%.
Table 2: Precision and Accuracy of the proposed method
| Taken BAC, c ·106 M | BAC added, c ·106 M | BAC Found, c ·106 M | Found BAC added*, (X̄ ±∆ X̄)·106 ,M | RSD, % | Recovery BAC added, % | t/t table |
|---|---|---|---|---|---|---|
| 2.80 | - | 2.82 | - | - | - | - |
| 2.80 | 2.80 | 5.72 | (2.85±0.10) | 2.80 | 100.7 | 1.40/2.78 |
| 2.80 | 4.20 | 7.30 | (4.25±0.06) | 1.13 | 101.2 | 2.33/2.78 |
| 2.80 | 5.60 | 8.35 | (5.65±0.08) | 1.07 | 100.9 | 1.85/2.78 |
* Mean of five measurements (P=0.95)
CONCLUSION
The conjugated system of two consecutive reactions - perhydrolysis of acetylcholine and the reaction of peracetic acid oxidation of p-phenetidine, is an analytical reaction that can be applied in the enzymatic kinetic spectrophotometric determination of BAC in «Optrex» eye drops. The proposed method is inexpensive, fairly rapid and sensitive. The analytical parameters, sensitivity, precision, accuracy, rapidity can recommend the proposed method as an alternative to other reported methods as an instrument for quality control of preservative of eye drops. RSD < 2.8%. The LOQ (20 % of the inhibition degree) = 1.9·10-6 mol L-1.