INTRODUCTION
Iodine is a topical effective agent against a wide spectrum of bacteria, viruses, fungi, and protozoa1. Its inherent insolubility in water was overcome by alcoholic iodine solution and Lugol’s Solution containing 5% elemental iodine and 10% potassium iodide that was found to be irritating to the eyes, skin and mucous membranes. Over the last century, scientists have developed a number of iodine preparations to overcome the adverse side effects of iodine. The objective was to avoid such incompatibilities without a significant loss of germicidal efficacy. As a result, iodophores, such as PVP-Iodine (PVP-I) that acts as a solubilizing agent for iodine were developed2,3.
PVP-I has been widely used on burned skin, mucosa and contains a high amount of iodine that can be absorbed4. Systemic iodine absorption is reported even with single wound irrigation; therefore repeated usage should be avoided in patients with renal impairment5. Systemic toxicity after the application of PVP-I in dogs was reported by Glick et al6. They concluded that during continuous PVP-I irrigation, free iodine is absorbed in significant amounts. In another study, iododerma was reported following topical PVP-I application7. Systemic iodine absorption associated with the use of preoperative ophthalmic 10% PVP-I was reported in 15.5% 8. Hypothyroidism has occurred in neonates after PVP-I application and also to the mothers during pregnancy or breast feeding9. In the other study, Balin and Pratt showed that dilute solution of PVP-I has a toxic effect on human fibroblast and caution should be used in the topical application of PVP-I on the open wound and prolonged contact with tissue should be avoided10. Based on the above-mentioned studies new iodine carrier should be introduced to overcome the adverse side effects of PVP-I and limitation of iodine systemic absorption. Polymeric micelles (PMs) are self-assembled core-shell nanostructures consisting of amphiphilic copolymers11that can enhance the therapeutic efficacy and minimize the systemic side effects of topically applied drugs. A micellar structure is formed in an aqueous medium if one segment of the block copolymer can provide inter-chain cohesive interactions sufficient for the micelle formation12. Critical features of the PMs as drug carriers, including particle size, stability, loading capacity and release kinetics of drugs, can be modulated by the structures and physicochemical properties of the constituent block copolymers. Also, nano-engineering of block copolymers might allow the preparation of PMs with integrated smart functions, such as specific-tissue target ability, as well as chemical or physical stimuli-sensitivity. Thus, PMs are nanotechnology-based carrier systems that might exert the activity of potent bioactive compounds in a site-directed manner, ensuring their effectiveness and safety in the clinical use13.
PMs could enhance the deposition of drugs in targeted sites of the skin in the normal and dermatological disorders14. Hydrophobic compounds such as Iodine can be encapsulated in the core of the PM15. For this purpose, PMs have been selected for the localization of iodine into the skin.
MATERIALS AND METHODS
Materials
Iodine was purchased from part Kimia pharmaceutical company. Tween 80, span 20, oleic acid, Cholesterol, Poloxamer 188 and PEG were obtained from Merck (Germany). Oleoyl polyoxyl-6 glycerides M 1944 CS (Labrafil) and propylene glycol monocaprylate TM 90 (Capryol) were a gift from Gattefosse (France). Hydroxyl propyl methyl cellulose (HPMC) (KM4) provided by Aldrich chemical company Inc. Povidone-Iodine 10% was a gift from Behvazan pharmaceutical company (Rasht, Iran). Dialysis bag was purchased from Armaghane Kalaye Gavan Company (Tehran, Iran).
Solubility of iodine
The solubility of iodine was determined in different oil (oleic acid, isopropyl myristate and paraffin) and surfactant (Labrafil, Tween and Span mixture) by dissolving an excess amount of iodine in 5ml of oil, and other components using a stirrer for 30 minutes at 45°C and then overnight at room temperature.
Determination of the critical micelle concentration (CMC)
Surfactant and co-surfactant aqueous solutions with different concentrations were prepared and the surface tension was measured at 25 ºC with a Torsion balance (WHITE ELEC Model NO. 83944E). Then the chart of surface tension versus log concentration was plotted. Critical micelle concentration (CMC) calculated based on this plot.
Preparation of iodine polymeric micellar formulation
Defined amounts of oleic acid and PEG 300 with cholesterol mixed together and then chloroform added to this mixture. 1% iodine dissolved in this mixture and put into a rotary at 120 rpm and 60 ºC for 15 min to forming uniform lipid film. Then 10 ml aqueous solution containing, polymer (HPMC or poloxamer), surfactant (labrafil or Tween 80: Span 20 1:1) and co-surfactant (capryol) added to uniform lipid film, and put the mixture in the sonicator with power 500w at 25 ºC and diluted by distilled water to 100 ml. Based on a full-factorial design with three variables on two levels 8 PMs formulation was prepared that their compositions illustrated in Table 1. Three independent variables as surfactant type (S), polymer type (P) and surfactant/ oil ratio (S/O) were used in this study. Two types of surfactants with different HLB have been selected for evaluating the effect of HLB on micelle stability and Iodine solubility in aqueous solution. Labrafil with HLB=4 demonstrates more lipophilic property than a mixture of Tween+Span with HLB=7.
Table 1. Different amount of compounds in the PMs (data are presented as w/v %)
| formulation Batch No | (S/O) ratio | S | Polymer | Oleic acid | cholesterol | PEG300 | Tween 80 | Span20 | capryol | poloxamer 188 | HPMC | Labrafil |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | Lab | P | 0.5 | 0.3 | 0.5 | - | - | 0.01 | 0.1 | - | 0.8 |
| 2 | 1 | Lab | h | 0.5 | 0.3 | 0.5 | - | - | 0.01 | - | 0.1 | 0.8 |
| 3 | 1 | T+S | p | 0.5 | 0.3 | 0.5 | 0.4 | 0.4 | 0.01 | 0.1 | - | - |
| 4 | 2 | Lab | p | 0.5 | 0.3 | 0.5 | - | - | 0.01 | 0.1 | - | 1.6 |
| 5 | 2 | T+S | p | 0.5 | 0.3 | 0.5 | 0.8 | 0.8 | 0.01 | 0.1 | - | - |
| 6 | 2 | Lab | h | 0.5 | 0.3 | 0.5 | - | - | 0.01 | - | 0.1 | 1.6 |
| 7 | 1 | T+S | h | 0.5 | 0.3 | 0.5 | 0.4 | 0.4 | 0.01 | - | 0.1 | - |
| 8 | 2 | T+S | h | 0.5 | 0.3 | 0.5 | 0.8 | 0.8 | 0.01 | - | 0.1 | - |
S/O: surfactant/oil ratio; P: poloxamer; h: HPMC (K4M) ; T: Tween; S: Span
Particle size measurements
Particle size was measured at 25 °C by particle size analyzer. The mean droplet size of samples was determined by SCATTER SCOPE 1 QUIDIX (South Korea) based on photon correlation spectroscopy with a wide measurable size range (1-7000 nm). Each sample was measured triplicate.
Iodide assay by high-pressure liquid chromatography (HPLC) method
Iodide ion (I-) was selected as a major species of iodine and determined by the HPLC method consists of the C18 column (5µm, 25 cm), acetonitrile: 50 mmol/l sodium dihydrogen phosphate buffer solution (50:50 v/v) mixture as mobile phase, UV-detector at 230 nm.
In-vitro Release study
Franz diffusion cells (area 3.4618 cm2) with a cellulose membrane (molecular weight cut off 3000-5000) were utilized to determine the release rate of iodine from different PMs formulations. The membrane was then clamped between the donor and receptor compartments receptor was filled with 22ml of distilled water. Iodine-loaded PMs (5cc) placed in the donor compartment. At 0.5, 1, 2, 3, 4, 5, 6, 7, 8 and 24 h time intervals, a 2 ml sample was removed from the receptor for iodide determination by HPLC and replaced immediately with distilled water.
Animal experiments
Male adult Wistar rats (weighing 150 - 170 g) with ages between 10 - 12 weeks were purchased from the animal laboratory, Jundishapur University of Medical Sciences, Ahvaz, Iran. Animals were kept for 5 days of acclimatization with a standard diet and were then treated according to the principles for the care and use of laboratory animals. Approval for the studies was given by the Ethical Committee of the Ahvaz Jundishapur University of Medical Sciences (grant number: N-67). The rats were anesthetized with sodium thiopental prior to sacrificing them. Abdominal full-thickness skin was removed and any extraneous subcutaneous fats cleaned from the dorsal side using cooled pure acetone solution.
Skin integrity was evaluated by using an electrical conductometer at 300 HZ by two stainless steel electrodes. Diffusion cells with a resistance lower than 3.9± 0.15 k Ώ cm-2 were rejected16.
In vitro skin permeation studies
The whole skin sample was mounted between the donor and receptor compartments of the vertical glass diffusion cells. Iodine-loaded PMs and PVP-I put in the donor compartment and the receptor cell was filled with phosphate buffer (pH 7) including 0.2% span 20. The diffusion cell was placed in a water bath 37 ± 0.5 oC equipped by a magnetic stirrer. At predetermined time intervals (0.5, 1, 2, 3,……,8, 24 h), a 2 ml sample was removed from the receptor and immediately replaced with phosphate buffer including 0.2% span 20 to maintain sink condition. At 24 h, the donor solutions were collected in order to calculate the iodide concentration. As a control, a blank cell without Iodine-loaded PMs and PVP-I were performed as the other cells. The results were plotted as cumulative permeated drug versus time. Based on these plots, the apparent permeability coefficient (Equation 1) and the steady-state permeation flux (Jss) (Equation 2) were calculated.
dQ/dt is the steady-state appearance rate on the acceptor side of the skin. A is the area of the skin (cm2) and C0 is the initial concentration of the drug in the donor phase. On the other hand, % of iodide un-permeated after 24 h (R24%) was calculated based on Equation 3.
On the other hand, the % of iodide into the skin after 24 h (D24%) was calculated by Equation 4.
Minimal inhibitory concentration (MIC) of iodine-loaded polymeric micelles and PVP-I
The MIC of 1% iodine-loaded PMs and commercial 10% PVP-I solution was determined by a microtiter plate using Mueller Hinton medium. Appropriate concentrations of the sample were prepared and tested against Gram-positive Staphylococcus aureus and Gram-negative Pseudomonas auroginosa that procured from Persian Type Culture Collection (PTCC), Tehran, Iran. Bacterial suspensions were adjusted to 0.08- 0.1 at 600 nm.
Two-fold serial dilution of (Iodine-loaded PMs and PVP-I) in Mueller Hinton was carried out to yield different concentrations. Equal bacterial inoculum (5µl) was transferred to each well and the plate was incubated at 37°C for 12-18 hours. All wells were evaluated for bacterial growth for the determination of MIC. Wells showing no bacterial growth were streaked on Mueller Hinton agar and incubated overnight for Minimal Bactericidal Concentration (MBC).
RESULTS
Solubility study
Among the used oils the oleic acid showed maximum solubility for iodine and also labrafil as a surfactant demonstrated high solubility capacity (Table 2). Therefore oleic acid was used as an oily phase and Labrafil and mixture of Tween 80+Span20 as surfactants.
Critical Micelle Concentration (CMC) determination
Amounts of CMC for surfactant, surfactant with co-surfactant and polymer are shown in Table 3. CMC was sensitive to polymer type. So poloxamer can produce micelle with the lower concentration that is an advantage for topical formulation. The result of this study was in accordance with the finding reported by Xu in 201317. They reported that polymers in comparison with surfactant can form micelle with lower CMC and therefore show more stability against dilution. But finding in this study demonstrates a direct correlation between CMC and the degree of hydrophobicity of the polymers. Poloxamer 188 is composed of a central hydrophobic chain linked by two hydrophobic chains of polyoxyethylene which has HLB around 318. HPMC is a non-ionic hydrophilic polymer that forming micelle in higher concentration19. The CMC of poloxamer is affected by other components in the formulation. For example, salts and urea Causes decreasing and increasing effects on CMC of poloxamer respectively, and sodium lauryl sulfate causes to form mix micelles20. Therefore in this study due to concomitant use of poloxamer with surfactants such as Labrafil and mixture of Tween and Span, it is clear that CMC will change.
PMs characterization
Particle size, percentage of drug released after 24 (DR 24%) and iodide content of PMs were calculated and demonstrated in Table 4.
Table 4. Different characters of PMs (Mean ±SD ، n=5)
| Formulation No | Particle size (nm) | DR24% | Iodide content (mg/ml) |
|---|---|---|---|
| 1 | 46.8±7.35 | 51.1±5.3 | 0.18±0.02 |
| 2 | 153±6.65 | 54.5±4.7 | 0.19±0.02 |
| 3 | 96±0.57 | 37.5±4.3 | 0.20±0.02 |
| 4 | 14.2±1.75 | 50.1±4. 2 | 0.19±0.02 |
| 5 | 91.3±3.95 | 38. 9±4.5 | 0.19±0.02 |
| 6 | 121.3±7.51 | 58. 7±6.7 | 0.20±0.02 |
| 7 | 111.1±5.15 | 64.7±6.3 | 0.19±0.02 |
| 8 | 134.6±6.64 | 63.5±5.2 | 0.19±0.01 |
| PVP-I | - | - | 0.43±0.02 |
Particle size
The particle size of formulations was between 14.2 to 153 nm. Regression analysis showed that among the independent variables, the relationship between polymer type and particle size was significant (P = 0.04) in this manner that by changing the polymer from HPMC with poloxamer significant reduction in particle size occurred.
Iodine content and in vitro drug release
Iodine content in all PMs was less than the PVP-I solution. The value of DR 24% is around 37.5 - 64.7. Based on these results PMs were prepared by poloxamer demonstrated lower values of DR 24% in comparison with HPMC.
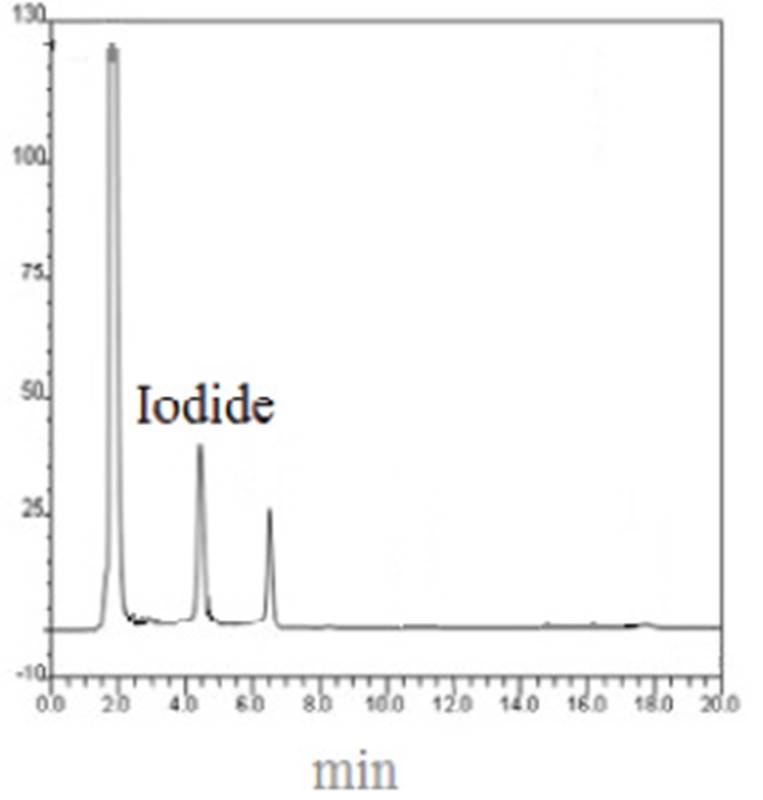
It seems that PMs act as a depot for iodine and release it slowly. For this reason, iodine content in PMs is lower than PVP-I. the chromatogram of iodide in HPLC is presented in Figure 1.
Permeability study
The drug permeability parameters through rat skin by different formulations and PVP-I are illustrated in Table 5. The values of Q24(mg of iodide permeated in 24 h) obtained by all PMs were lower than these values obtained by PVP-I. This parameter is affected by iodide concentration. Iodide concentration in PVP-I is more than iodide concentration in PMs. For this reason judgment about the effect of PMs based on Q24is associated with the error. But D 24%is not dependent on concentration. Results show PMs 1, 3, 4 and 5 demonstrated higher D 24%than PVP-I (P-vale<0.05). But other PMs indicated lower values of D 24% significantly (P-vale<0.05) compared PVP-I. Formulations 1, 3, 4 and 5 are made by poloxamer as mucoadhesive polymer. Formulation 1 indicated the highest amount of D 24%and cumulated the highest amount of iodide into the skin. Poloxamer with bioadhesive property increased iodine residence time into the skin. Only formulations 1, 4 and 5 decreased significantly Jss compared with PVP-I that formulation 1 with the highest effect indicated 3 folds decrease in Jss.
Table 5. Different permeability parameters of iodide through rat skin after application of iodine-loaded PMs and PVP-I (Mean ± SD, N=5)
| Formulation no | R24(mg) | Q24(mg) | D24% | JSS(µg/cm2.h) |
|---|---|---|---|---|
| 1 | 0.24 ±0.031 | 0.19±0.02 | 52.2±5.8 | 2.3±0.18 |
| 2 | 0.102±0.002 | 0.75±0.05 | 12.62±1.1 | 9.1±1.01 |
| 3 | 0.18±0.013 | 0.514±0.06 | 31.3±3.9 | 6.2±0.68 |
| 4 | 0.33 ±0.046 | 0.232±0.03 | 41.8±4.7 | 2.8±0.35 |
| 5 | 0.28±0.03 | 0.431±0.03 | 24.8±1.83 | 5.2±0. 50 |
| 6 | 0.12±0.01 | 0.847±0.093 | 6.5±0.74 | 10.2±1.03 |
| 7 | 0.11±0.008 | 0.714±0.07 | 13.2±0.95 | 8.6±0.66 |
| 8 | 0.33±0.041 | 0.531±0.04 | 8.2±0.73 | 6.4±0.68 |
| PVP-I | 0.42±0.035 | 1.73±0.23 | 19.5±2.3 | 20.8±3.05 |
In vitro MIC and MBC of Iodine-loaded PMs and PVP-I
The values of MIC and MBC according to PVP-I and Iodine-loaded PMs for pseudomonas auroginosa and Staphylococcus aureus are presented in Figure 2. All PMs produced MIC against pseudomonas around 7.8-8.4 µg/ml that didn’t show a significant difference with PVP-I. This value is in accordance with the MIC that previously reported by Houang et al21. The MIC and MBC values provided by all PMs against Staphylococcus aureus were comparable with PVP-I and are higher than values reported previously22. No significant correlation between independent variables and values of MIC and MBC against two microorganisms was found. It means that the PMs didn’t alter the activity of Iodine against both microorganisms.
DISCUSSION
Toxicity with iodine after application of PVP-I was reported in human. This toxicity, especially after application on wound and mucosa, indicated high iodine absorption through the skin. The aim of this study was the design and evaluation of the capacity of PMs to decrease the rate and amount of iodine permeation through the skin. PMs demonstrated particle size between 14-153 nm which is affected by the CMC and molecular weight of the polymer. PMs with 1% iodine included 0.18-0.210 mg/ml Iodide as major species. But PVP-I with 10% iodine consisted of 0.435 mg/ml iodide. Iodide/iodine ratios for PMs and PVP-I are 0.018-0.021 and 0.0043, respectively. This contrast indicates that more iodide is produced by PMs. Therefore the capacity of PMs for iodine dissolving is more than PVP-I. For providing equal available iodine with PVP-I, 1.5% iodine- loaded PM solutions should be produced. 3% iodine liposomal formulation that was introduced by another study23indicated lower iodine potency in compare with PMs that introduced in the present study. PMs presented iodine controlled release patterns that mainly affected by the type of polymer. Polyethylene oxide chain in poloxamer is responsible for the lipophilic property wich increased iodine affinity for loading and lower drug release. PMs significantly decreased the values of Jss and Q24 compared to PVP-I. In an ex vivo study, Jss of iodine after the application of PVP-I through human skin was reported 0.73 µg/cm2.h 4. The reason for higher Jss through rat skin in comparison with human skin is the lower barrier property of rat skin. Iodide concentration into the skin that is described by D24% determines antimicrobial efficacy of topically applied antiseptics including iodine. Results showed PMs made by poloxamer increased D24 % compared PVP-I. Formulation 1 showed the best property in iodine localization into the skin and reduction in iodine percutaneous absorption. This formulation had the lowest particle size that facilitated iodine penetration into the skin. On the other hand simulation systems with water-lipid bilayer interface indicated that hydrophilic blocks of poloxamers interact with lipid headgroups and remain at the interface and hydrophobic blocks enter into the hydrophobic region of the bilayer18. These simulations for poloxamer micelles next to the lipid bilayer showed no permeation of these micelles into the bilayer. Therefore poloxamer micelles do not penetrate into the skin bilayers and localize iodine on the skin.
CONCLUSION
The aim of this study was to the reduction of iodine permeation through the skin. The poloxamer micelles demonstrated low CMC, small particle size and slow release profile. Poloxamer micelles indicated good iodine loading and solubilization properties that decreased iodine dose in the micelles. Poloxamer micelles decreased in vitro skin and increased iodine skin concentration compared to PVP-I. on the other hand, PMs have MIC and MBC values comparable with PVP-I. Therefore poloxamer micelles can be introduced as appropriate iodine carriers instead of PVP-I.