Introduction
Liposomes are spherical, self-enclosed vesicles in which aqueous part is entirely enclosed by phospholipid bilayer and liposomes is amphiphilic in nature, means it possess both lipophilic and hydrophilic character1. Liposomes are prepared from a variety of natural and synthetic phospholipids, and considered as drug-carrying structures or vesicles2.
Liposomes, which are versatile, biodegradable, nontoxic phospholipid vesicles, are known to reduce toxicity associated with many compounds including antitumor3, antibiotics4, and immunosuppressive agents5.The reasons for using liposomes can be summarized as follows: liposomes can enhance cellular internalization of the drug; they can generally decrease unwanted systemic toxic effects; and they may increase drug solubility in biological fluids, modulating at the same time the drug release profile. Furthermore, in some case, the use of phospholipid vesicles to carry the drug to target cells could possess specific release by passive or active targeting strategies6.
Controlled drug release can be achieved by transdermal drug delivery systems (TDDS) which can deliver medicines via the skin portal to systemic circulation at a predetermined rate over a prolonged period of time. TDDS has gained a lot of interest during the last decade as it offers many advantages over the conventional dosage forms and oral controlled release delivery systems notably avoidance of hepatic first pass metabolism, less frequency of administration, reduction in gastrointestinal side effects and improves patient compliance7,8.
Drug was chosen because of its low meting point, low partition coefficients and optimum range of molecular weight which is prerequisite for development of transdermal drug delivery system8.
Transdermal therapeutic systems are defined as a self-contained, discrete dosage forms which, when applied to the intact skin, deliver the drug, through the skin at control rate to the systemic circulation. Transdermal formulation maintain drug concentration within the therapeutic window for prolong period of time ensuring that drug levels neither fall below the minimum effective concentration nor exceed the maximum effective concentration9.
Baclofen (BCF) is a direct agonist at GABAB receptors. Baclofen used to relax the muscle during the condition of muscle contraction10. The purpose of the current study was to investigate the feasibility of liposomes to formulate the transdermal administration of baclofen.
Materials and Methods
Baclofen was a provided as gift sample from Sun Pharmaceutical Industries Limited, India. Soya lecithin was purchased from Acros Organics, New Delhi, India. Cholesterol was purchased from Sigma, USA. Stearic acid purchased from Research Lab, Mumbai India. Ethanol (AR), Methanol and Chloroform purchased from Merck, India. The all other materials used in this study were of analytical grade.
The baclofen liposomes prepared as per method reported A. Kumar, et al., (2010) with slight modifications. For the preparation of liposome various formulations were prepared using different ratio of lecithin, cholesterol and ethanol but drug and stearic acid ratio kept constant (Table 1). The lecithin, cholesterol, stearic acid and drug were dissolving in organic solvents (ethanol) and injected in to 10 ml preheated PBS water at 55-65oC with continuous stirring at 500 rpm using magnetic stirrer. The solvent was evaporated by heating so as to obtain drug loaded liposomes11.
Vesicle size:
The size of liposomes was determined by using a film of vesicle dispersion, fixed with 10% w/v gelatin solution, was observed under a light microscope (BEM-21, Besto Microscope, India) fitted with an ocular micrometer and stage micrometer at a magnificationof 100X12.
Vesicle shape:
Vesicles morphology of baclofen liposomes obtained from ethanol evaporation was determined using a photomicroscope (Nikon Model UFX-II, Japan) at 1000 x magnification12.
Entrapment efficiency:
The lipoosomal dispersions were centrifuged (90 XL Ultracentrifuge, Beckman, USA) at 10,000 × g for 20 min to separate unentrapped drug and washed with phosphate buffered saline (pH 7.4). The clear supernatant was analyzed for baclofen by UV-spectrophotometer (Shimadzu UV-1700, Japan) at 226 nm. The amount of entrapped drug was calculated using the following equation13.
In vitro drug release study:
The liposomes encapsulating baclofen were separated by gel filtration on sephadex G-50 column which was kept in double distilled water for 10 h for swelling. Then the prepared liposomal suspension (1mL) was placed on the top of the column and elution was carried out using normal saline. The liposomes loaded with baclofen elutes out first as a slightly dense, white opalescent suspension, were followed by free drug. Separated liposomes were filled in a dialysis tube to which a dialysis sac was attached to either end. The dialysis tube was suspended in phosphate buffered saline (pH 7.4) at 37±2°C, stirred with a magnetic stirrer and samples were withdrawn at specific time intervals and analyzed spectrophotometrically (λmax 226 nm). The volume was replenished with the same amount of fresh dissolution fluid each time to maintain the sink condition14.
Stability studies:
The liposomal formulations were subjected to stability studies by storing at 4±2°C, 25±2°C and 37±2°C in thermostatic oven for 6 month15. Then drug content of all the formulations was determined in terms of % entrapment at reported temperature.
In vivo study:
The apparatus consists of a horizontal wooden rod or metal rod coated with rubber with 3 cm diameter attached to a motor with the speed adjusted to 30 rotations per minute. The rod is 40 cm in length and is divided into 3 sections by plastic discs, thereby allowing the simultaneous testing of 45 mice. The rod is in a height of about 50 cm above the table top in order to discourage the animals from jumping off the roller. Cages below the sections serve to restrict the movements of the animals when they fall from the roller. The experiments were performed on male Swiss albino mice weighing 20-25 g. The mice were trained to run on the rod rotating at a rate of 10 rpm for 3 days. Only those animals which have demonstrated their ability to remain on the revolving rod for at least 300 s were used for the test. Two or three trials were usually enough for the animals to learn this task. The formulation as well as plane drug was applied topically with the help of custom designed plastic ring. Then it was allowed to be in contact with mouse skin for 30 minutes to ensure proper absorption. The mice were placed for 5 min on the rotating rod. The number of falls from the roller during this time was counted. Diazepam (2 mg/kg, i.p.) was used as positive control16. The data of number of falls were analyzed by one way ANOVA followed by Student-Newman-Keuls test.
Vesicle size:
Particle size determination of liposomes was performed by light microscope after storage. The size of different formulations of baclofen liposome was found to be in the range of 3.98±0.45-4.24±0.65 µm.
Vesicle shape:
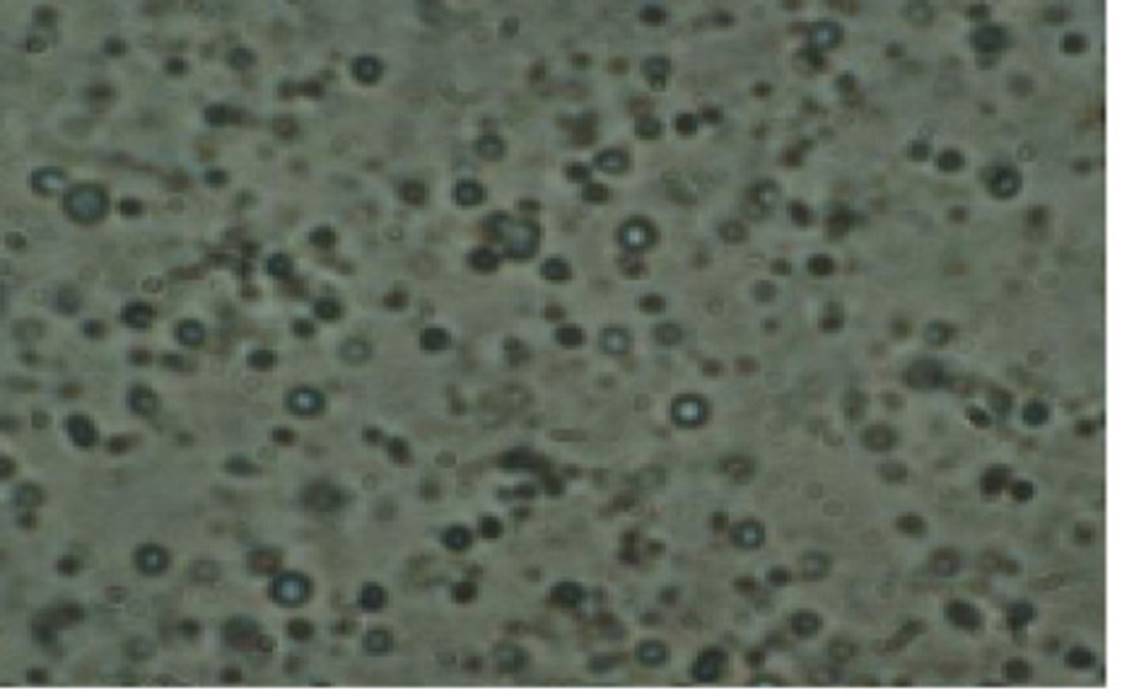
The microscopic study of drug loaded liposomes shows that small unilamellar vesicles were formed by ethanol injection method. Microscopic observations of the prepared liposome formulation confirmed the formation of spherical particles as shown in Figure 1.
Entrapment efficiency:
The entrapment efficiency of all the baclofen liposome formulations (RLP1, RLP2, RLP3, RLP4 and RLP5) was carried. The formulation RLP5 of baclofen liposome shows maximum % entrapment efficiency (58.67±0.81 %) compared to RLP1, RLP2, RLP3 and RLP4 formulations (Table 2).There was increase in % entrapment efficiency was observed with an increase in ethanol concentration.Improvement in aqueous solubility of baclofen was achieved with higher concentration of ethanol, due to its co-solvent effect. The % entrapment efficiency also increased with an increase in concentration of lecithin.
Table 2: Effect of lipid content on drug entrapment efficiency (results are ±SD, n=3)
| Formulation Code | % EE |
|---|---|
| RLP1 | 40.13±0.41 |
| RLP2 | 44.32±0.56 |
| RLP3 | 50.63±0.15 |
| RLP4 | 55.58±0.17 |
| RLP5 | 58.67±0.81 |
In vitro drug release study:
The in vitro drug release study of baclofen liposomes formulation was carried out up to 10 h in PBS (pH 7.4) at 37±2°C. The formulation RLP5 of baclofen liposome shows maximum % cumulative drug release (67.66±5.32 %) compared to RLP1, RLP2, RLP3 and RLP4 formulations (Table 3). Formulation RLP5 shows maximum % cumulative drug release due to the maximum entrapment of drug in the vesicles. This was apparently low in magnitude (pure drug-about 92 % vs. RLP5-67 %) and released over longer period of time as compared to pure drug taken as standard. Therefore, the drug release could be said to be in sustained fashion. As the cholesterol ratio increased the % cumulative drug release also increased but afterwards it shows diminished release of drug because vesicles consist of several concentric sphere of bilayer above the aqueous compartment. Stearic acid also helps to promote the drug contents release from the vesicles because of its softening and lubricating behaviors.
Table 3: Effect of cholesterol, lipid and stearic acid on in vitro% release profile of baclofen liposome in PBS 7.4 pH, 37±2°C at λ max 226 nm (results are ±SD, n=3)
| Time (h) | % Cumulative drug release | |||||
|---|---|---|---|---|---|---|
| Pure drug* | RLP1 | RLP2 | RLP3 | RLP4 | RLP5* | |
| 0 | 00±0.00 | 00±0.00 | 00±0.00 | 00±0.00 | 00±0.00 | 00±0.00 |
| 1 | 24.26±2.14 | 10.34±1.23 | 12.71±5.21 | 13.34±6.12 | 15.35±1.22 | 16.24±1.45 |
| 2 | 32.54±3.42 | 14.56±3.58 | 15.81±4.13 | 16.89±5.12 | 17.35±3.25 | 19.46±3.12 |
| 3 | 39.35±1.67 | 20.74±1.45 | 22.47±2.33 | 24.46±2.66 | 23.83±3.45 | 26.89±8.45 |
| 4 | 46.87±7.18 | 25.89±4.78 | 27.85±1.36 | 31.43±2.22 | 30.34±2.33 | 32.64±5.55 |
| 5 | 58.15±6.44 | 28.61±8.23 | 31.18±3.45 | 38.18±9.13 | 39.49±0.88 | 38.49±5.61 |
| 6 | 66.89±7.54 | 35.42±2.43 | 36.69±4.82 | 44.67±6.43 | 47.91±6.65 | 45.82±3.67 |
| 7 | 74.73±9.45 | 41.45±6.41 | 43.68±3.12 | 51.49±0.98 | 53.81±4.58 | 52.91±7.17 |
| 8 | 81.95±1.32 | 50.32±5.78 | 47.59±3.54 | 59.43±2.13 | 56.84±1.26 | 61.87±3.21 |
| 9 | 88.65±9.34 | 57.57±8.34 | 58.62±1.32 | 62.87±3.22 | 60.43±6.33 | 65.57±2.36 |
| 10 | 92.75±6.32 | 62.67±5.32 | 63.65±7.81 | 65.78±2.44 | 64.61±1.89 | 67.66±5.32 |
*Statistically significant difference in drug release (paired ‘t’ test at p=0.05)
Stability studies:
For stability studies liposome formulations were stored at different temperature such as 4±2 °C, 25±2°C and 37±2°C. The maximum % entrapment was observed in 4±2 °C (refrigerated) temperature compared to 25±2°C and 37±2°C temperature (Table 4). The magnitude of drug retention within the vesicles, on storage under defined conditions, ultimately governs the shelf life of the developed formulation. Acceleration in drug leakage at higher temperatures, as observed in storage-stability studies, suggested keeping the liposomal product in the refrigeration conditions, to minimize the drug leakage from liposomal-systems. The optimized formulation RLP5 shows better storage stability at refrigerated condition than other formulations.
Table 4: Stability study of different formulations of baclofen liposomes
| Formulation Code | Time (month) | ||
|---|---|---|---|
| 1 | 3 | 6 | |
| Temperature (°C) | |||
| 4±2 | |||
| % Entrapment | |||
| RLP1 | 57.61±0.91 | 56.82±0.34 | 56.72±0.43 |
| RLP2 | 57.65±0.82 | 58.68±0.56 | 58.62±0.51 |
| RLP3 | 58.31±0.45 | 59.98±0.42 | 60.83±0.67 |
| RLP4 | 62.22±0.54 | 61.65±0.31 | 61.62±0.74 |
| RLP5 | 64.19±0.26 | 63.54±0.68 | 63.45±0.23 |
| 25±2 | |||
| RLP1 | 46.48±1.36 | 45.78±0.71 | 46.56±0.28 |
| RLP2 | 46.78±0.86 | 47.67±0.15 | 48.59±0.61 |
| RLP3 | 49.67±0.81 | 50.62±0.43 | 49.23±0.13 |
| RLP4 | 55.38±0.46 | 52.56±0.71 | 51.34±0.35 |
| RLP5 | 58.84±0.91 | 55.52±0.87 | 53.69±0.41 |
| 37±2 | |||
| RLP1 | 38.77±0.18 | 37.89±0.12 | 37.89±0.71 |
| RLP2 | 40.88±0.35 | 39.67±0.24 | 38.26±0.18 |
| RLP3 | 41.36±0.78 | 40.57±0.45 | 41.91±0.40 |
| RLP4 | 44.37±0.71 | 42.51±0.69 | 43.59±0.51 |
| RLP5 | 47.74±0.21 | 46.48±0.58 | 46.73±0.30 |
In vivo study:
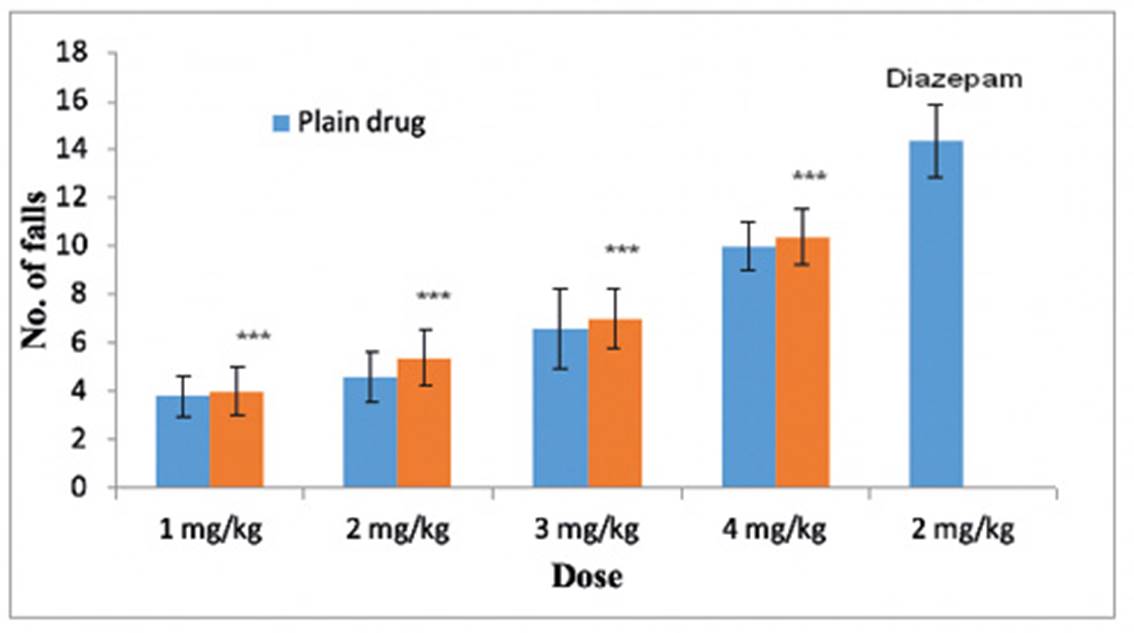
In vivo study was carried out to check the potential activity of formulation, and it was determined by rota-rod apparatus at CT Institute of Pharmaceutical Sciences, Jalandhar (Punjab), India. For the in vivo study 45 mice (Mus musculus) were selected. The dosage regimen was 1 to 4 mg/kg for optimized formulation RLP5 and plane drug as per literature. The plane drug treated (1, 2, 3 and 4 mg/kg) mice showed lesser number of falls from rota-rod apparatus in comparison to optimized formulation RLP5 (1, 2, 3 and 4 mg/kg) and diazepam (2 mg/kg), results are given in Table 3.1. Similarly optimized formulation RLP5 treated mice showed increased number of falls than plane drug treated mice but lesser number of falls was observed in case of diazepam treated mice. When the dose of optimized formulation was increased (1, 2, 3 and 4 mg/kg) there was increase in number of falls from rota-rod apparatus, because liposomal formulation permeated well through the skin and absorbed in systemic circulation, and may completely relax the muscle. The mice treated with formulations have shown improved muscle relaxant activity which was evident by increased number of falls in rota-rod test as compared to plane drug treated mice. The data pertaining to number of falls were analyzed by one way ANOVA followed by Student-Newman-Keuls test. The effect of formulation was dose dependent (p values are 0.001 for 1, 2, 3 and 4 mg/kg, respectively). However, diazepam treated mice may resulted higher muscle relaxation than any dose of formulation tested (Figure 2).
Conclusions
Liposomal formulation containing baclofen possessed particle size, particle shape, entrapment efficiency, in vitro drug release, stability and in vivo studies and on the basis of above these studies revealed that due to small size and spherical shape of liposomes gaining access from the epidermal layer of the skin to effectively deliver the baclofen. Liposomal formulation releases the baclofen for longer period of time in a sustained release manner. Liposomal formulations stable at 4±2°C temperature because in accelerated temperature more drug leakage occurs. Baclofen loaded liposomal formulation shows skeletal muscle relaxant activity performed by rota rod method in mice which could deliver the baclofen from liposomes in the therapeutic range. For transdermal drug delivery of baclofen via liposomes could deliver and entrap lesser quantity of drug for the treatment of spasticity. Finally concluded, the formulation RLP5 of baclofen liposome shows better performance than those of respective formulations.