Introduction
The factors involved in the inflammatory process are present in many pathologies. The search of anti-inflammatory agents is one of the most addressed fields in the research of new therapeutic targets1,2. Although there is a considerable therapeutic arsenal of anti-inflammatory synthetic drugs, the high incidence of adverse events has determined a lasting searching for drugs of natural origin with similar pharmacological effectiveness and low toxicity index3.
Saponins are secondary metabolites from numerous species with structural similarity to steroidal anti-inflammatory drugs4 5 6-7. Agave brittoniana subsp. brachypus (Trel.) A. Álvarez, (Asparagaceae) endemic specie to the Cuban central region and belonging to the genus Agave8, is characterized by its high content of steroidal saponins. Studies in different biological systems have shown the anti-inflammatory activity of several species of the genus Agave9. Agave sisalana Perrine showed an anti-inflammatory effect in carrageenan-induced rat paw edema and cotton pellet-induced granuloma10. An anti-inflammatory effect has been reported from Agave angustifolia on croton oil-induce ear edema in mice.11. There is a study of da Silva and Parente about Agave brittoniana specie from Brasil that establishes the anti-inflammatory properties of a steroidal saponin extracted from its leaves12. In the present study we show the results of a different subspecie, that despite being reported to grow up in our country, it contains Δ5 saponins13, unlike those studies from Brazilian researchers who described a 5α spirostan saponin with carbonil group attached at the C-12 position.
The previous evidences do not show enough experimental studies about the anti-inflammatory activity in vivo of steroidal saponins from a butanolic fraction of leaves of Agave brittoniana subsp. brachypus. Thus, the aim of the present study was to assess the anti-inflammatory effect of a saponin-enriched fraction from Agave brittoniana subsp. brachypus (Trel.) A. Álvarez, (Asparagaceae) in experimental animals.
Methods
A phytochemical study of saponin-enriched fraction from A. brittoniana leaves was carried out to confirm the presence of metabolites with pharmacological potential. The butanolic extract obtained was evaluated in acute and chronic experimental models of inflammation.
The A. brittoniana leaves were collected in the morning hours in Cubanacan protected area, nearby to the Santa Clara city. The voucher specimen was identified in the Botanic Garden of UCLV (HPVC # 5445). Healthy leaves were selected, without mechanical, chemical or microbiological detectable damage.
Drying and milling
To facilitate drying, the collected plant material was finely cut. The plant was dried for 7 days at 40 °C in oven (Binder, Germany). The dried material was milled on a cutting miller (IKA-MF 10B, Germany) at 3000 rpm. The product was sieved through a 3 mm internal diameter sieve.
Organoleptic properties and numerical indexes of the dry drug
Smell and color of the dry drug were recorded. The numerical indexes, total ash and moisture residual content were determined to the dried and milled material, (Public Health Branch Standard 309: NRSP 309). The determination of moisture residual content was made by the gravimetric method14.
Obtaining saponins from crud extract
The extractive process described by Guerra and several researchers in order to obtain saponins-enriched extracts was done.15 16-17 First, a hydroalcoholic extract was obtained: 300 g of the dry and milled plant material was macerated with 1 L of 70% ethanol and protected from light. The menstruum was reconstituted in alternate days until total discoloration of the mixture. Then, a dark green mixture was obtained by rotoevaporation at 40 °C (RotoevaporadorIKA® RV 10 basic).
Afterwards, 50 g of the hydroalcoholic extract was resuspended in one portion of water and diluted in 180 mL of distilled water. The mixture was placed in a separatory funnel, adding 180 ml of n-butanol. It was previously saturated with water, and the extractions were made every 24 hours for three days. Two fractions were obtained, one organic, containing the crude saponins, and another aqueous. Finally, the n-butanolic fraction was rotoevaporated toward total dryness.
Phytochemical screening
To corroborate the composition of the n-butanolic fraction, phytochemical screening was carried out following the technique described by Miranda and Cuéllar18.
Acute model of carrageenan-induced rat paw edema
It was carried out according to the method described in CYTED19. Male Sprague-Dawley rats of initial weight between 180-200 g were used. Food was removed three hours before administration. Doses of 25, 50 and 100 mg/kg of the n-butanolic fraction or saponin-enriched fraction were applied orally. The control group received only the vehicle (distilled water) and the positive control received indomethacin as an anti-inflammatory agent (7 mg/kg p.v.). One hour after the administration of the product, 0.1 mL of a solution of carrageenan (1%) in physiological saline was administered into the plantar aponeurosis of the right hind paw of each rat. The thickness of the leg was measured with a tape measure before inducing the edema and at one, three, four and five hours after injection with carrageenan.
The inhibition percentage of the inflammatory reaction of γ-carrageenan was calculated at one, three, four and five hours of the injection, using the following formula:
Chronic model of cotton pellet-induced granuloma
The technique described by Sheth and updated by other researchers was used 20,21. Prior to the experiment, the cotton discs were weighed (average weight 16 ± 2 mg) and sterilized in an autoclave. The animals were anesthetized with ketamine hydrochloride (50mg/kg). Sterilized cotton pellet were surgically inserted in the axillary region of each rat through a single needle incision.
The next day group I (negative control) received distilled water (10 mL/kg). Groups II, III and IV received doses of 25, 50 and 100 mg/kg of the n-butanolic fraction, respectively. Group V (positive control) received solution of indomethacin (5 mg/kg). This oral administration was repeated for seven days at a rate of 10 mL/kg. On the eighth day, the animals were sacrificed by ether overdose followed by cervical dislocation, taking into account the Good Laboratory Practices22.
The granulomas formed were extracted and weighed on an analytical balance. Then, they were dried in the oven for two hours at 60 °C and weighed again to determine their dry weight, which corresponds to the amount of fibrogranulomatous tissue formed in the chronic stage of inflammation.
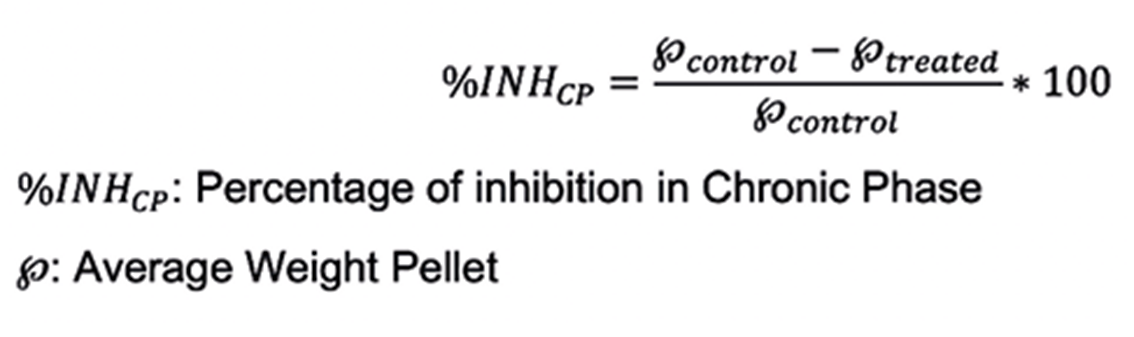
The corresponding inhibition percentage was determined according to the following formula:
Statistical analysis
The data was processed by the statistical package SPSS for Windows Version 20.0. Mean and standard deviation of the parameters evaluated were determined. Differences between the parameters obtained in each experimental group were made using the ANOVA and t-student parametric test and the non-parametric tests of Kruskal Wallis and Mann-Whitney, in both cases with a confidence interval of 95%.
Results
Phytochemical screening
The qualitative evaluation of the phytochemical composition of the n-butanolic fraction is show in Table 1.
Table 1. Secondary metabolites in n-butanolic fraction of A. brittoniana
| Metabolite | Assay | Result |
|---|---|---|
| Alkaloids | Dragendorff | - |
| Mayer | - | |
| Wagner | - | |
| Triterpens and Steroids | Liebermann-Burchard | + |
| Quinones | Borntrager | - |
| Coumarins | Baljat | - |
| Essentials Oils and Fats | Sudan III | - |
| Reducer sugars | Fehling | +++ |
| Saponins | Espuma | +++ |
| Phenols and Tanins | Iron Chloride (III) | - |
| Aminoacids and Amines | Nihidrina | - |
| Flavonoids | Shinoda | - |
| Glycosides | Molish | - |
| Cardiotonic Glycosides | Kedde-Raymond | - |
+Low +++ High
These results demonstrate the success of the followed procedure to extract steroidal saponins to a butanolic fraction starting from a hydroalcoholic extract of A. brittoniana leaves.
Acute model of carrageenan-induced rat paw edema
Figure 1 illustrates the evolution of the paw´s thickness during five hours. As can be seen, all the treated groups had less value in the indicator paw´s thickness than the control without treatment. A dose-dependent effect, with an area under the curve less than indomethacin in the group of 100 mg / kg butanolic fraction is also found.
The average paw´s thickness and % INHAP calculated for each group at different time are showed in Table 2.
Table 2. Anti-inflammation effect in acute model of carrageenan-induced rat paw edema
| Average Paw´s Thickness (mm) | ||||
|---|---|---|---|---|
| 1h | 3h | 4h | 5h | |
| nBF25 | 20.50±12.86b(54.44) | 29.67±8.41b (36.43) | 21.33±9.83b(48.80) | 21.33±11.69c (46.67) |
| nBF50 | 25.00±10.12b(44.44) | 21.67±6.71b (53.57) | 10.83±6.05b(74.00) | 11.67±6.71b (87.83) |
| nBF100 | 19.83±9.50b(55.93) | 20.67±8.04b(55.71) | 6.00±4.55c(70.40) | 4.80±3.76b (81.87) |
| INDOM | 41.67±4.08a (7.41) | 21.67±9.83b(53.57) | 14.00±5.48b(76.00) | 6.00±5.48b (91.67) |
| CTROL - | 45.00±10.49a | 46.67±10.33a | 41.67±7.53a | 40.00±6.32a |
aThe reported values between parentheses represent the % INHAP. nBF: n-butanolic fraction in doses of 25, 50 and 100 mg/kg, INDOM: indomethacin
bDifferent font means statistical differences in the same column (p < 0.05, Man Whitney Test)
The statistical analysis of the paw thickness variation of treated animals showed significant differences from the first hour after the administration of the inflammatory inductor (Kruskal Wallis, p = 0.01). All groups of the n-butanolic fraction showed lower values of paw´s thickness than those observed in the control group from the first hour. The anti-inflammatory effect of indomethacin was observed afther three hours. All groups of saponin-enriched fraction behaved similarly to this anti-inflammatory drug (Mann-Whitney, p > 0.05). At four hours anti-inflammatory effect of the saponin-enriched fraction was observed, highlighting that 100 mg/kg dose presented higher inhibition than the indomethacin. The % INHAP of medium and high doses of n-butanolic fraction had kept similar values to the indomethacin group, at five hours.
Chronic model of cotton pellet-induced granuloma
Table 3 shows the fibro granulomatose content and the percentage of inhibition of experimental groups.
Table 3. Anti-inflammatory effect in a chronic model of cotton pellet-induced granuloma
| GROUPS | Fibrogranulose Content (mg) | % INHCP |
|---|---|---|
| nBF25 | 9.58 ± 3.32a | 53.72 |
| nBF50 | 11.57 ± 1.71a | 45.76 |
| nBF100 | 8.96 ± 4.21b | 56.40 |
| INDOM | 14.33 ± 4.06a | 53.66 |
| CONTROL | 22.7 ± 4.98c | - |
anBF: n-butanolic fraction in doses of 25, 50 and 100mg/kg, INDOM: indomethacin
bDifferent font means statistical differences in the same column (p < 0.05, t-student Test)
The one-way ANOVA test indicated significant differences in the weight of the fibrogranulose content. The group without treatment presented values of 22.7 ± 4.98 mg of fibrogranulose content, significantly higher than all the treated groups (p > 0.05, t student). The highest dose of n-butanolic fraction registered even lower values than the indomethacin group, while the rest of doses presented statistically similar values to this group (p = 0.05, t student). The percentage of the inhibitory inflammation, according to the dry content, showed values very similar to the indomethacin, with a slightly higher value in the group of 100 mg/kg of saponin-enriched fraction.
Discussion
The saponins from crude extract obtained with low contamination of other metabolites coincide with reports of extraction of saponins in other species following the same extraction protocol23 24-25. Former studies about A. brittoniana carried out by Guerra and collaborators stated that the best method to obtain saponin-enriched leaves extracts from this species is by using serial extractions of ethanol first and butanol through a separatory funnel later, as well as we used in this study.15,16
The steroidal saponins, metabolites of interest in A. brittoniana, should share, from their chemical structure, pharmacological properties similar to corticosteroids. These endogenous molecules have an anti-inflammatory effect due to their ability to stabilize the lysosomal membrane, to reduce capillary permeability and the releasing of bradykinin and histamine. The corticosteroids stimulate the synthesis of lipocortins, inhibitory proteins blocking the phospholipase A2 synthesis and then affecting eicosanoid synthesis26,27.
Previous studies indicate that one hour after the administration of γ-carrageenan; histamine and serotonin have a major role as mediators. Approximately, at two hours the kinins intervene. Between three and four hours after induction, the maximum vascular response coincides with the phase mediated by the prostaglandins PGE1, PGE2 and PGF228,29.
The data suggest that the saponins extracted from the plant exert their activity by inhibiting the release of the first mediators. This situation illustrates how in the acute model of carrageenan, a decrease in inflammation with respect to the negative control group and indomethacin is observed from the first hour. Several studies relate that saponins could contribute to the membrane´s stabilization, in this phase, by decreasing capillary permeability30,31.
After three hours the activity increases, which is seen in the abrupt fall of the inflammation values (Figure 1). In this phase, the production of prostaglandins is activated. As it has been already indicated, the structural similarity of the steroidal saponins with the endogenous steroids allows assuming the inhibition of the eicosanoid synthesis, preventing the release of inflammatory prostaglandins. Indomethacin, a drug inhibiting the cyclooxygenase and the synthesis of prostaglandins, has its greatest activity after three hours, which coincides with other authors32.
An increase in the recruitment of macrophages, lymphocytes and different substances closely related to the immune response, can be observed in the proliferative phase of chronic model of cotton pellet-induced granuloma33. Steroidal saponins, as corticosteroids, could reduce the number of inflammatory cells, including eosinophils, T lymphocytes, mast cells and dendritic cells, in different tissues. Then, these secondary metabolites could decrease the size of the granuloma by suppressing the growth of collagen fiber7.
Saponins, such as escin, have an anti-inflammatory effect in chronic model of cotton pellet-induced granuloma, possibly by inhibition of signal molecules related to the glucocorticoid receptor34. Agave americana species have been studied in both acute and chronic inflammation models. In the second, the presence of saponins is responsible for the decrease in granuloma´s dry weight, which means their anti-inflammatory activity in this model35.
Finally, an aspect to highlight is the known structural composition of the saponins present in A. brittoniana. Their aglycone coincides with sapogeninas such as diosgenin and yuccagenin13,16,17. Several studies have shown that diosgenin inhibits the production of several proinflammatory interleukines, as well as the generation of free radicals, decreasing the macrophages’ recruitment36. Saponins have a non-negligible intestinal metabolism, which allows the steroidal ring to be absorbed and passed into the systemic circulation where it could reach the target tissues37. Yuccagenin, a major sapogenin in this species, is like diosgenin with an additional hydroxyl group en position two38. For that reason, it is necessary to conduct other studies to evaluate the anti-inflammatory contribution of yuccagenin present in A. brittoniana.
The results allow us to conclude that the saponin-enriched butanolic fraction from the hydroalcoholic extract of Agave brittoniana could have the major contribution of anti-inflammatory effect of this specie, comparable to indomethacin in acute and chronic models. Subsequent research could indicate which saponins or sapogenins found in the plant are the ones with the greatest contribution to this activity.