Introduction
Bladder cancer is the twelfth most common cancer worldwide1. The highest incidence rates occur in Europe, being relatively low in South and Central America2. Due to the high recurrence rates and the clinical and cytopathological follow-up of patients’ surveillance, bladder cancer treatment is one of the most expensive, generating a major economic burden on health-care systems3. Approximately 90% of malignant bladder tumors are represented by urothelial cell carcinomas4. Moreover, TP53 mutations are the most common alterations in bladder cancer cells and are frequently detected in advanced disease states5.
Currently, several natural substances have been investigated as alternative approaches for treating diseases such as cancer6,7. The allyl isothiocyanate (AITC), the major compound of mustard seeds, has been considered a promising antineoplastic agent against several types of cancer, including bladder cancer8-10. In spite of its potential activity, the application of AITC is restrained by some characteristics, including poor aqueous solubility, instability at high temperature, and susceptibility to degradation by nucleophilic molecules11.
Micellar systems have been extensively studied for the administration of anticancer agents, because they are capable to incorporate hydrophobic substances in the hydrophobic core shell, increasing the solubility of the drugs and protecting them from in vivo inactivation12. Moreover, the micellar systems allow the passive delivery of the encapsulated substance to the tumor by the increased permeability and retention effect13.
In view of the potential of AITC as an antineoplastic agent and the benefits of micellar systems to delivery substances, the aim of this study was to develop Pluronic® F127 micellar solutions containing AITC and evaluate their antineoplastic activity in bladder tumor cell cultures.
Methods
Materials
Pluronic® F127 (F127), AITC, Dulbecco's modified Eagle's medium (DMEM), penicillin G, streptomycin, Tween 20 and propidium iodide were purchased from Sigma-Aldrich® (St Louis, USA). Acetonitrile (HPLC grade) was purchased from J. T. Baker® (Xalostoc, Mexico). Fetal bovine serum was purchased from Cultilab Ltd. (Campinas, Brazil). Cell Proliferation Kit II (XTT) was purchased from Roche Diagnostics® (Mannheim, Germany). Culture medium DMEM without phenol red was purchased from Invitrogen® (Carlsbad, USA). Giemsa stain was purchased from Dinâmica® (Diadema, Brazil).
Preparation of micellar solutions
Briefly, the micellar solutions containing AITC were prepared by the cold dispersion method14. A weighed amount of F127 (10 and 15% wt/wt) was added to a becker containing ultrapure water and kept under moderate magnetic stirring in an ice bath until complete dispersion of the polymer. The solution was kept in refrigerator for 24 hours to allow the complete dissolution of the polymer. Subsequently, the AITC was added and dispersed under vigorous stirring at room temperature. The final AITC concentration was 12.5 µM. Micelles without AITC (controls) were prepared using the same method.
Preparation of free AITC solution
The free AITC solution was prepared by dissolving 1.22 µL of AITC in a 2% Tween 20 solution prior to its use.
Determination of mean size and zeta potential of the micelles
The particle size and zeta potential values of micelles were determined by Zetasizer (Malvern, model Zetasizer Nano series - Nano ZS) at 25°C. The mean particle size was measured based on photo correlation spectroscopy technique. The zeta potential was determined based on electrophoretic mobility measurements. The experiments were conducted in triplicate.
Encapsulation efficiency in micellar solutions
The amount of AITC present in the micellar solutions was determined by HPLC/UV method as previously described15. The equipment used was the Waters e2695 coupled to a UV/Vis Waters 2485 detector. The UV/Vis detector was set at 242 nm. Separation was done on a C18 column (Phenomenex, Luna 5μ, 100Å, 150 x 4.6 mm) at 25ºC by using acetonitrile and ultrapure water (70:30) as mobile phase at a flow rate of 1 mL/min. The injection volume was 25 uL and the run time for AITC was approximately 8.5 minutes.
Cell lines
The human urothelial carcinoma cell lines RT4 (from a low grade tumor with the wild type TP53 gene) and T24 (from a high grade tumor with the TP53 allele encoding an in-frame deletion of tyrosine 126) were purchased from the Cell Bank of the Federal University of Rio de Janeiro (Rio de Janeiro, Brazil). The cell cultures were culture in DMEM supplemented with 10% bovine fetal serum, 100 U/ml penicillin G, 100 U/ml streptomycin and maintained in an atmosphere of 5% CO2 at 37ºC.
Cytotoxicity and cell proliferation
Cytotoxicity and cell proliferation assays were performed using the Cell Proliferation Kit II (XTT). Briefly, cells were seeded into 96-well plates (1×104 and 1.5×104 cells/well for T24 and RT4 respectively). After 24 hours, the cells were treated with different concentrations (0.005, 0.0625, 0.0725, 0.0825, 0.0925, 0.125, 0.25, 0.5 µM) of the micellar solutions containing AITC or with free AITC during three hours. The concentrations and the time of treatment were defined based on the study conducted by Sávio and colleagues10. Cells treated with micellar solutions without AITC and untreated cells were used as controls. After treatment, the cells were washed with Hank's solution (0.4 g KCl, 0.06 g KH2PO4, 0.04 g Na2HPO4, 0.35 g NaHCO3, 1 g glucose and 8 g NaCl in 1 L·H2O). After washing, fresh culture medium was added and the cells were incubated for 21 (cytotoxicity) and 45 hours (cell proliferation) in independent experiments. Afterwards, 12 uL of XTT test solution (1 mL XTT labeling solution/20 uL of electron-coupling reagent) were added to each well and the absorbance was measured at 492 and 690 nm after 90 minutes. Absorbance results are proportional to the percentage of viable cells. Tests were conducted in triplicate.
Cell Morphology
The formulation MS15+AITC was chosen to continue the study, since it presented satisfactory results at cytotoxicity and cell proliferation assays, adequate particle size, monodisperse index parameters and retained the greater amount of AITC (99%) after preparation.
Initially, 2 × 105 cells were seeded into 12-well plates. After 24 hours, the cells were treated with MS15+AITC (0.0725 µM) for three hours. As controls, cells treated with micellar solutions without AITC and untreated cells were used. After the treatment, the cells were washed with Hank's solution, fresh culture medium was added and the cells were incubated for 21 hours. Afterwards, the cells were observed under a phase-contrast microscopy at 200x magnification and the alterations were photographed.
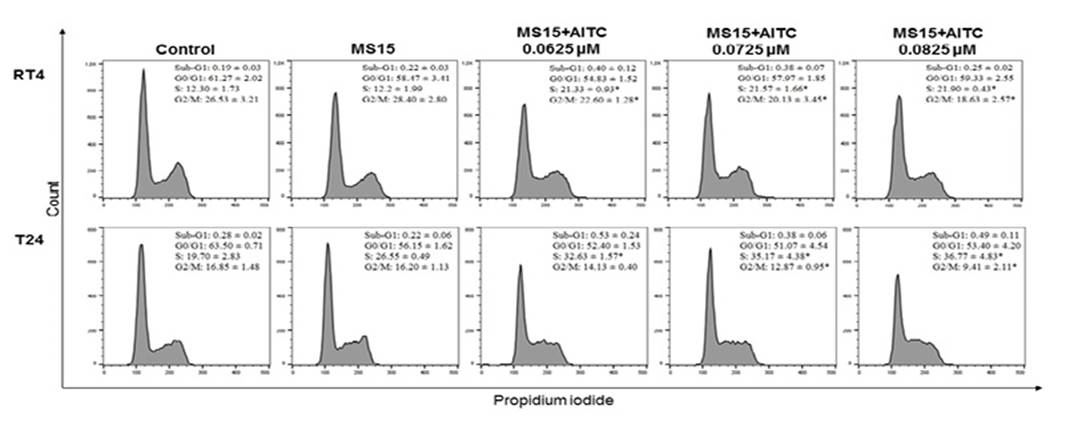
Cell cycle kinetics
For cell cycle kinetics, 2 × 105 cells were seeded into 12-well plates. After 24 hours, the cells were treated with MS15+AITC at concentrations 0.0625, 0.0725 and 0.0825 uM for three hours. Cells treated with micellar solution without AITC and untreated cells were used as controls. After the treatment, the cells were washed with Hank's solution, fresh culture medium was added and the cells were incubated for 21 hours. Afterwards, cells were detached using trypsin and centrifuged at 1000 rpm for 10 minutes. The sediment was fixed with 70% ethanol and maintained at -20°C for 12 hours. Subsequently, the cells were washed, resuspended in 200 µL of labeling solution (0.0914 g of magnesium chloride; 0.0774 g of sodium citrate; 0.04766 g of Hepes; 10 uL of Triton-X, 0.5 mL of propidium iodide, 9.490 mL of water), placed on ice and protected from light for at least 30 minutes16. The percentages of cells in the G0/G1, S and G2/M phases were measured using flow cytometry (BD FACSCalibur) and analyzed using FlowJo® software. 30.000 events were analyzed and the experiments were conducted in triplicate.
Clonogenic survival
Clonogenic assay was used for evaluating the long-term effects of micellar solutions. Briefly, cells were plated at a density of 1×106 cells/25 cm3 culture flask. After 24 hours, the cells were treated with MS15+AITC at concentrations 0.005, 0.0825 and 0.25 uM for three hours. Cells treated with micellar solution without AITC and untreated cells were used as controls. Cultures were rinsed with Hank's solution, trypsinized and approximately 1×103 cells were plated into 25 cm3 culture flasks and allowed to grow for 15 days to form colonies. The cells were Giemsa stained and the number of colonies with 50 or more cells was counted. The experiments were performed in triplicate.
Results
Determination of particle size and zeta potential of micelles
The results of the particle size and zeta potential of the micellar solutions are shown in Table 1. The micelles containing AITC exhibited size greater than the micelles without AITC. The particle size distribution of MS10+AITC and MS10 micelles was polydisperse while to the MS15+AITC and MS15 micelles was monodisperse. The zeta potential of the micellar solutions containing AITC was negative and smaller (in absolute value) than the micelles without AITC.
Encapsulation efficiency in micellar solutions
The Table 1 shows the encapsulation efficiency of AITC in the micelles. It was observed that about 97% and 99% was loaded in MS10+AITC and MS15+AITC micelles, respectively.
Table 1. Mean particle size, polydispersion index, zeta potential and percentage of AITC encapsulation
| Size (nm) | PI | Zeta potential (mV) | % of encapsulation | |
|---|---|---|---|---|
| MS10+AITC | 44.7 ± 6.18 | 0.65 ± 0.07 | - 4.81 ± 2.31 | 97.0% |
| MS15+AITC | 88.1 ± 25.46 | 0.28 ± 0.01 | - 10.80 ± 5.13 | 99.0% |
| MS10 | 5.3 ± 0.27 | 0.42 ± 0.03 | - 11.20 ± 4.64 | - |
| MS15 | 6.3 ± 3.19 | 0.27 ± 0.11 | - 12.30 ± 5.14 | - |
The values represent the means ± standard deviation. MS10+AITC: 10% F127 + AITC; MS15+AITC: 15% F127 + AITC; MS10: 10% F127; MS15: 15% F127, AITC: allyl isothiocyanate; PI= polydispersion index.
Cytotoxicity and cell proliferation
As shown in Figure 1-A, increased cytotoxicity rate was observed only after treatment with the highest concentration of MS15+AITC tested (0.5 μM) in T24 cells. No significant decrease in cell viability was observed after treatment with MS10+AITC or free AITC.
Figure 1-B shows significant reduction of cell viability only after treatment with MS15+AITC at 0.5 μM in RT4 cells. Moreover, increased citotoxicity rates after treatment with MS10+AITC at concentrations above 0.0925 μM were observed. No decrease in cell viability was detected after treatment with free AITC.
Forty eight hours after treatment, T24 cells showed significant decrease of cell proliferation at all concentrations tested of MS10+AITC (except 0.0625 and 0.0725 μM) and at 0.005, 0.0625, 0.0725, 0.125, 0.25 and 0.5 μM of MS15+AITC. Inhibited cell proliferation was not observed after treatment with free AITC (Figure 2-A). To RT4 cells, all concentrations of MS10+AITC and MS15+AITC significantly inhibited the cell proliferation. Treatment with free AITC reduced the cell proliferation only at concentrations of 0.25 and 0.5 μM (Figure 2-B).

Figure 1. Percentage of viable T24 (A) and RT4 (B) cells (logarithmic scale) 24 hours after treatment with free AITC and micellar solutions contain AITC. MS10+AITC: 10% F127 + AITC; MS15+AITC: 15% F127 + AITC. * p < 0.05 compared to the control. Each point represents the mean values ± standard deviation obtained from three experiments.

Figure 2. Percentage of viable T24 (A) and RT4 (B) cells (logarithmic scale) 48 hours after treatment with free AITC and micellar solutions contain AITC. MS10+AITC: 10% F127 + AITC; MS15+AITC: 15% F127 + AITC. * p < 0.05 compared to the control. Each point represents the mean values ± standard deviation obtained from three experiments.
Cell Morphology
The phase-contrast photomicrographs of the RT4 and T24 cells showed a lower number of cells after treatment with MS15+AITC. Additionally, elongated T24 cells after treatment were observed (Figure 3).
Cell cycle kinetics
For RT4 and T24 cells, significant increase in the number of cells in the S phase accompanied by a decreased number of cells in the G2/M phase was detected after treatment with MS15+AITC (Figure 4).
Clonogenic survival
The result of the clonogenic survival assay showed significant decrease of number of colonies in RT4 and T24 cell culture after treatment with 0.0825 and 0.25 uM MS15+AITC and with free AITC (Figure 5). No significant difference was observed between the free AITC and the MS15+AITC treatment in relation to number of colonies.
Discussion
The AITC selectivity has already been demonstrated. According to Bhattacharya and colleagues17, AITC did not induce significant cell cycle arrest and apoptosis rates in normal cells at the concentrations that were highly effective against cancer cells. It was suggested that AITC may be delivered more readily to bladder cancer tissue than to the normal bladder tissue. The normal bladder epithelium has protective mechanisms, which include tight junctions, thickened apical membrane and coverage by a mucopolysaccharide layer. Probably, these protective barriers do not exist in the bladder cancer cells18. The use of a micellar formulation could further improve this selectivity of AITC since micelles allow the passive delivery to tumor cells by the increased permeability and retention effect13. Based on the information available of AITC effects on normal bladder cells17, this study aimed to understand the mechanisms of action of micelles containing AITC on bladder tumor cells with different TP53 gene status. Attempts to correlate tumor chemoresistance with TP53 status have shown that the therapeutic response depends on the type of TP53 mutation and the treatment used19.
F127 is a tri-block copolymer constituted by two hydrophilic and one hydrophobic chain that self-aggregates in aqueous solutions to form reversible amphiphilic polymeric micelles. The hydrophobic part of F127 form the core where hydrophobic substances can be encapsulated, being isolated from the external aqueous environment by the hydrophilic shell formed by the hydrophilic part of the molecule20. Polymeric micelles have a critical micelle concentration (CMC) that is the lowest concentration limit for polymers to produce a micelle21. According to Stammet and colleagues22, the F127 CMC is 2.8 µM. When diluted below CMC, polymer micelles are gradually disintegrated into unimers and this can affect the solubilizing efficacy of the formulation. For this reason, it was chosen to use the quantities of F127 (10 and 15% w/w or 7936.5 and 1190.0 µM, respectively) that even after dilutions present concentrations higher than F127 CMC.
AITC is a volatile substance and its dispersion by the cold method in F127 micellar solution could prevent the loss of the drug by evaporation, since it was observed that over 97% of the AITC was retained in the formulations. The incorporation of AITC into F127 micelles was also evidenced by the mean size increase, which suggests that the drug was partitioned into the hydrophobic core of the micelles, increasing the volume.
The size of the micelles is particularly important to their administration. Size variations within the nanometer scale can affect blood circulation time and the bioavailability of the particles into the body23. During the tumor growth occurs the development of new vessels in a process known as angiogenesis. These vessels are generally characterized by a discontinuous endothelium with large fenestrations of 200-780 nm, allowing to the passage of nanoparticles, different from blood vessels of healthy tissue that have smaller fenestrations24. For the treatment of cancer, the desirable size of a nanocarrier is 10 to 100 nm25. Thus, the small size of AITC micelles (< 90 nm) can become another important advantage to the possible use of these formulations for bladder cancer treatment.
The zeta potential of the micelles was negative in module, before and after incorporation of AITC. It is well known that Pluronics® can provide steric stabilization to the colloidal systems26. Therefore, the micellar systems can possess good stability since F127 polymer is able to stabilize the micelles against aggregation by steric interactions, even if zeta potential values were lower than -30 mV.
The cytotoxicity data demonstrated that 10% and 15% F127 micellar solutions containing AITC were able to potentiate the cytotoxic effect of the drug 24 hours after treatment, since no significant reduction in viability of cells treated with free AITC was detected. It is suggested that the AITC, a hydrophobic substance, is dispersed in the medium and captured by the cells when encapsulated in F127. The ability of the polymeric micelles to increase the aqueous solubility is due to their hydrophobic core that provides a suitable microenvironment to accommodate hydrophobic substances23.
Our results showed that lower MS10+AITC concentrations were more cytotoxic to RT4 (IC50 0.06195 µM) than T24 (IC50 3.7345 µM) cells, 24 hours after treatment. Free AITC also was more cytotoxic to RT4 (IC50 0.2197 µM) than T24 (IC50 2.3125 µM) cells. Influence of TP53 mutations in the cellular responses to chemotherapy is still poorly understood, because it depends on a complex signaling cascade. However, it is noticed that cells carrying the mutated TP53 gene, as T24 cells, are more resistant to chemotherapy because of the role this gene plays in the control of apoptosis27,28.
After 48 hours of treatment, it was observed that F127 micelles containing AITC induced a decrease in cell proliferation at lower concentrations than those observed 24 hours after treatment, for both cell lines. This finding may suggest that the concentrations AITC-contained F127 micelles could lead to a decreased cell reproduction capacity, probably by a sustained lethal damage. Moreover, decreased cell proliferation rates were independent of TP53 status. Sávio and colleagues10 found increased rates of apoptosis after 48-hour AITC treatment in RT4 and T24 cells, suggesting that AITC, probably, is able to induce apoptosis through TP53 independent and dependent pathways.
The morphological alterations found in T24 cells and the low cell density in both cell cultures after MS15+AITC treatment are suggestive of cell cycle arrest29. Several studies have demonstrated the ability of free AITC to induce cell cycle arrest in G2/M phase in different types of tumor cell lines, including breast adenocarcinoma, colorectal adenocarcinoma, glioma and bladder tumor cells10,30-32. Differently, we showed that MS15+AITC induced cell cycle arrest in S phase in both cell lines. Thus, it is suggested that the encapsulation of AITC in micelles changes the mechanism of action of this compound regarding the modulation of cell cycle, probably interfering with the DNA replication33. On the other hand, free AITC is able to bind to cysteine residues and α- and β-tubulins, promoting their degradation and inducing cell cycle arrest in mitosis34.
The long-term effects of free AITC and AITC-containing F127 micelles were evaluated by the clonogenic survival assay. This assay is used to evaluate the ability of a cell to proliferate indefinitely, retaining its reproductive capacity after being exposed to a substance35. The results demonstrated that both free AITC and AITC-containing F127 micelles were able to generate a lethal damage with loss of the reproductive potential to both bladder tumor cells. Thus, these results indicate the formulation was able to maintain the long-term effects of free AITC.
Therefore, the AITC-F127 micellar solutions could become an interesting approach for the treatment of bladder tumor, considering that this delivery system could not only maintain the long-term effect of the drug, preventing the undesirable effects of administration of the free AITC, but also to accelerate the initial antitumoral effect of AITC by interfering in the cell cycle.