Highlights
Present study exhibited the chemical nature and the medicinal properties of Sonneratia apetalaBuch-Ham leaf habitat in Indian Sunderban. It contained small polyphenolic compounds and has strong antioxidant and antiulcer properties. It may lead to the development of effective compound(s) for the treatment in gastric ulcers, particularly in alcohol addicted population.
Introduction
It has been accepted that 75% of world’s mangroves located in just 15 countries mainly in Asia, Africa, North and Central America1. Indian Sunderban having the highest taxa diversity of mangrove with 69 species, 49 genera, 35 families2. Sonneratia apetala Buch-Ham (local name Keora) is predominant mangrove in Indian Sunderban. The bark, root, leaves and fruits have been used in folk medicine in the South Asian countries including India for treating diarrhoea, hepatitis, inflammation, wounds, ulcers3-6. Several bioactive constituents including fatty acids, triterpenoids and sterols from the aerial parts of S. apetala have been obtained7-8. Sadhu et al. (2006)9 reported S. caseolaris leaves have two flavonoids with antioxidant activities. Antioxidants defend against oxidative stress caused by necrotic agents are important factors to defend the gastric mucosa10. Plant derived antioxidants, especially phenolic acids and flavonoids have been extensively studied for anti-ulcerogenic efficacy11. There is little information on the pharmacological activities of S. apetala. Perhaps, alcohol consumption has been considered one of the most important factors to aggravate gastric ulcer in humans. For this purpose, we studied the role of Sonneratia apetala leaves in oxidative stress and focused on its active ingredients and protection against alcohol induced gastric ulcers in rats.
Methodes
Animals
Adult Wistar strain male albino rats, average body weight 175-180 g were procured from the registered animal breeder (M/s Saha Enterprise, Kolkata, Regn No. 1828/PO/Bt/S/15/CPCSEA) and used for this study. Principles of laboratory animal care guidelines were strictly followed12. The animals were maintained under 12:12 hours dark: light cycle, controlled temperature (22±3°C), with balanced diet and water ad libitum. Institutional Animal Ethical Committee (544/c/PO/02/CPCSEA) approved the experimental protocol. All the experiments were performed between 08:00-16:00 h.
Test drug preparation
The leaves of Sonneratia apetala Buch.-Ham were collected from the mangrove forest area of Indian Sunderban, West Bengal and identified by Botanical Survey of India, West Bengal. A voucher specimen (DST/537/5G-4/09-05) was deposited in the departmental herbarium. The shade dried and powdered leaves of S. apetala were extracted with hydro-methanol (20-80%) in soxhlet apparatus and concentrated by distilling the solvents and dried under reduced pressure. The extractive value was determined by gravimetric method and expressed as percentage yield. The bioactive phytoconstituents presences in hydro-methanolic extract of S. apetala (SA) were evaluated13. In brief, presence of alkaloids in SA was examined by Dragendorff’s test, sterols and triterpenoids by Liebermann-Burchard’s test, saponins by Froth test, tannins by lead acetate test, carbohydrates by Fehling’s test, reducing sugars by Benedict’s test, proteins by Millon’s test, phenolics by ferric chloride test, flavonoids by aluminum chloride test and glycosides by Borntrager’s test.
HPTLC Standardization
Gallic acid (3,4,5-trihydroxybenzoic acid), quercetin (5,7,3/,4/ tetrahydroxy flavonol) and coumarin (1,2-benzopyrone), three known biomarkers were used to standardize the extract14. SA was diluted with methanol (1 mg/ml) and spotted in the form of bands over a pre-coated silica gel plates (Merck, 60F254, 20x20 cm) using Camag Linomat 5 applicator. The plates were developed in the solvent system (toluene:ethyl acetate: formic acid = 4.5:3:0.2) for 30 min. The densitometric scanning was performed on Camag TLC Scanner 3 at 280 nm. The amount of gallic acid, quercetin and coumarin present in SA was calculated against respective standards and expressed per mg of extract.
Estimation of phenolic
Total phenolic in SA was determined as described earlier14. In brief, to 0.2 ml of SA solution (1 mg/ml methanol), 1.0 ml of Folin-ciocalteu reagent was added and incubated in dark for 5 min. Then, 0.8 ml sodium carbonate solution (7.5%) was mixed and kept in dark for 30 min at room temperature. Finally, 3 ml of deionized water was added and read at 765 nm (Ultrospec 2000 UV-visible spectrophotometer, Pharmacia Biotech, USA). Total phenolic content was expressed as gallic acid equivalent (GAE) in µg per mg of extract.
Estimation of flavonoids
Aluminum chloride method was employed for flavonoids estimation15. In brief, 0.2 ml of SA (1 mg/ml methanol) was added to 0.8 ml methanol, 0.1 ml of 10% aluminum chloride, 0.1 ml of 1 M potassium acetate and 2.8 ml of deionized water and read after 30 min at 415 nm and expressed as quercetin equivalent (QE) in µg per mg of extract.
Estimation of reducing power
The reducing power of SA was evaluated as described earlier14. In brief, 0.1 ml of SA was mixed with 2.5 ml of phosphate buffer (0.2 M, pH 6.6), and 2.5 ml of 1% potassium ferricyanide and incubated at 50°C for 20 min. Then, 2.5 ml of 10% Trichloroacetic acid was added to the mixture and centrifuged at 3000 rpm for 10 min. The upper layer of the solution (2.5 ml) was mixed with deionized water (2.5 ml) and ferric chloride solution (0.5 ml, 0.1%) and read at 700 nm. Reducing power was expressed as ascorbic acid equivalent (AAE) in µg per mg of extract.
Estimation of DPPH radical scavenging
The radical scavenging activity of SA was determined using the stable radical DPPH (1,1-diphenyl-2-picrylhydrazyl)15. In brief, 0.1 ml of SA in methanol at different known concentrations was mixed with 3.9 ml of DPPH solution (0.135 mM) and read after 30 min at 517 nm. Butylated hydroxyl toluene (BHT) was used as positive control. The sample concentration required to scavenge 50% of DPPH (IC50) was determined.
Estimation of superoxide anion radical scavenging
The incubation mixture contained 3 ml of Tris-HCl buffer (0.1 M, pH 7.4), 0.75 ml of nitroblue tetrazolium (300 µM) solution, 0.75 ml of NADH (936 µM) solution and 0.3 ml of SA at different concentrations (10-100 µl in methanol). The reaction started after adding 0.75 ml of phenazine methosulphate (120 µM) to the mixture. The reaction mixture was exposed to light at room temperature for 5 min and thereafter, read at 560 nm14. L-Ascorbic acid was used as positive control. The percent inhibition of super oxide anion generation of SA was calculated and was expressed as IC50.
Estimation of nitric oxide radical scavenging
Test extract (10-100 µl in methanol) was mixed to 0.5 ml of 1 M phosphate buffer saline, 2 ml of 10 mM sodium nitroprusside and kept in room temperature for 150 min. Potassium nitrite solution was used as standard. After incubation, 0.5 ml of the reaction mixture containing nitrite was transferred and mixed with 1 ml of 0.33% sulphanilic acid reagent and allowed to stand for 5 min for completing diazotization. Then, 1 ml of 1% naphthylethylene diamine dihydrochloride was added and read after 30 min at 540 nm16.
Estimation of hydroxyl radical scavenging
The reaction mixture contained 0.1 ml deoxyribose (2.8 mM), 0.1 ml ferric chloride (0.1 mM), 0.1 ml EDTA (0.1 mM), 0.1 ml hydrogen peroxide (1 mM), 0.1 ml ascorbate (0.1 mM), 0.1 ml phosphate buffer (20 mM, pH 7.4) and 1 ml of SA (10-100 µl in methanol). The mixture was incubated at 37°C for 1 h. At the end of the incubation period, 1.0 ml thiobarbituric acid (1%) was added and heated at 95°C for 20 min. After cooling, the thiobarbituric acid reacting substances (TBARS) formation was measured at 532 nm14. The percent TBARS production for positive control (H2O2) was fixed at 100% and the relative percent TBARS was calculated for the extract treated groups.
Estimation of ABTS radical scavenging
In brief, stock solution included ABTS (2,2’-azino-bis-3-ethyl benzthiazoline-6-sulphonic acid) solution (7 mM) and ammonium persulphate (2.45 mM) and working ABTS+ solution was then prepared by mixing the two stock solutions in equal quantities and allowing them to react for 12-16 h at room temperature in the dark. The final working ABTS+ solution was obtained after appropriate dilution with methanol (OD 0.7 at 734 nm). SA (10-100 µl in methanol) was added to 1 ml of ABTS+ solution and after 7 min read at 734 nm15. The percent inhibition was calculated and expressed as IC50.
Antiulcer activity in rats
Antiulcer activity of SA was evaluated on alcohol-induced gastric ulcers in rats as described by Morimoto and coworkers (1991)17 with slight modification18-19. Omeprazole (20 mg/kg, p.o) was used as standard prototype agent. All rats (body weight 150-175 g) were divided into five groups (N=6) as follows: Group I (normal control), Group II (treated control), Group III (SA 125 mg/kg), Group IV (SA 250 mg/kg) and Group V (Omeprazole 20 mg/kg). All animals were pre-treated according to above protocol for 10 days by oral route. On day 10, 50% alcohol at the dose of 5 ml/kg was administered orally to all rats (except normal control). Normal control rats were received distilled water (5 ml/kg). The last dose of all test drugs was given 1 h prior to alcohol administration. All animals were sacrificed under anesthesia (sodium pentothal 40 mg/kg, i.p) two hours after alcohol ingestion. The stomach was incised along the greater curvature and examined for gastric lesions. The gastric ulcer was scored and indexed as follows20.
Ulcer Index = (number of lesions I) + (number of lesion II) X 2 + (number of lesion III) X 3
I=presence of edema, hyperemia and single submucosal punctiform haemorrhages
II=presence of submucosal punctiform hemorrhagic lesions with small erosions
III=presence of deep ulcer with erosions and invasive lesions
Moreover, gastric mucosal tissues were dissected out and a portion was homogenized in cold phosphate buffer (0.1 M, pH 7.2), centrifuged and used for estimation of glutathione, catalase, lipid peroxides and protein19-22.
Statistical analysis
Data have been summarized by routine descriptive statistics. All statistical analyses were performed using statistical software package (SPSS, version 17.0, IBM, USA). Differences between and within groups have been assessed for statistical significance by standard parametric and non-parametric tests, as appropriate. ‘P’ value < 0.05 was taken as level of statistical significance in all tests.
Results
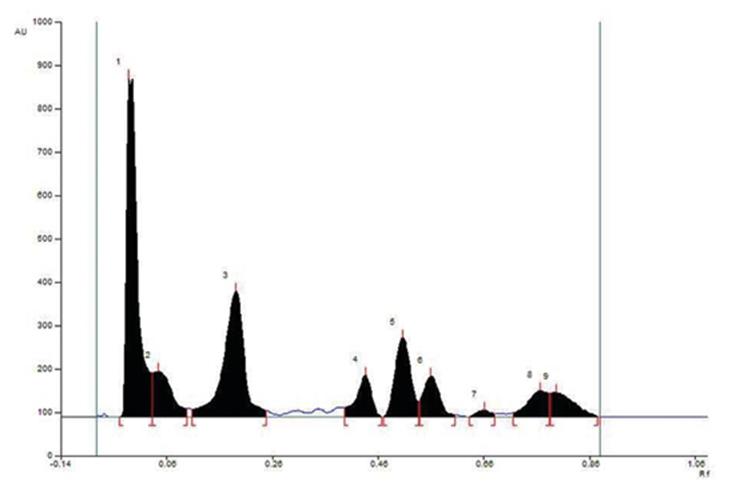
Chemical standardization. Presence of major bioactive phytoconstituents like, phenolics, flavonoids, triterpinoids, steroids and tannins are observed in SA. The yield or extractive value was found to 14.9%. As shown in Table 1, SA contains high amount of phenolics acids and flavonoids. Moreover, HPTLC chromatogram shows distinct peaks for gallic acid (peak 3), quercetin (peak 4) and coumarin (peak 5) in SA (Fig. 1). Rf values of gallic acid, quercetin and coumarin are 0.20, 0.42 and 0.51 respectively.
Table 1. Chemical constituyentes in Sonneratia apetala
| Phenolics (mg GAE/mg extract) | Flavonoids (mg QE/mg extract) | HPTLC densitometric quantification (µg/mg extract) | |||
|---|---|---|---|---|---|
| Gallic acid | Quercetin | Coumarin | |||
| SA | 7.37 ± 0. 501 | 7.41 ± 0.209 | 31.77±0.654 | 20.52±0.080 | 55.13±0.243 |
Values are mean±SE of triplicate samples; SA:hydro-methanolic (20:80) extract of Sonneratia apetala leaves; GAE:gallic acid equivalent; QE:quercetin equivalent
Antiradical activities. In vitro antiradical properties of SA are exhibited in Table 2. The mangrove extract exhibits strong reducing abilities, DPPH radical scavenging activity and cation (ABTS+) inhibitory properties. The extract also inhibits nitric oxide generation, superoxide radical formation and hydroxyl radical activities in a dose dependant manner.
Table 2. In vitro antioxidant activities of Sonneratia apetala
| Assay/Test | IC50 (mg/ml SA extract) |
|---|---|
| Reducing power | 156.61±3.50 |
| DPPH+ scavenging | 0.81±0.01 |
| NO radical scavenging | 2.04±0.03 |
| O2 ●scavenging | 2.79±0.02 |
| HO● scavenging | 1.42± 0.08 |
| ABTS+ scavenging | 0.16±0.003 |
Mean ± SE of triplicate samples; SA: hydro-methanolic (20:80) extract of Sonneratia apetala leaves; IC50:50% inhibitory concentration
Antiulcer activity. There are significant differences in gastric mucosal damage score among alcohol induced control, Omeprazole (20 mg/kg) and SA (125 and 250 mg/kg) treated groups (Table 3). Further, compare with the alcohol group, SA pre-treatment shows a dose dependent protection from ulcer lesion similar to standard Omeprazole. Moreover, SA pre-treatment significantly and dose dependently reduce the elevation of lipid peroxides, while enhance the concentration of glutathione and catalase in gastric mucosa in respect to alcohol induced control. Omeprazole also diminishes lipid peroxidation and enhances the level of glutathione and catalase in gastric tissues.
Table 3. Anti-ulcer action of Sonneratia apetala leaves
| Groups | Dose (p.o) | Ulcer Index | Gastric tissue | ||
|---|---|---|---|---|---|
| Lipid peroxides (nM MDA/mg protein) | Glutathione (nM/mg protein) | Catalase (mM of H2O2 decomposed/min/mg protein) | |||
| Normal control | 5ml/kg | - | 0.18±0.012 | 1.75±0.04 | 2.27±0.036 |
| EtOH | 5ml/kg | 33 ± 1.57 | 0.43 ± 0.014a*** (138.88) | 0.67 ± 0.02a*** (-61.71) | 0.58 ± 0.039a*** (-172.41) |
| SA+EtOH | 125mg/kg | 23.8 ± 1.13b** (-27.87) | 0.36 ± 0.016b* (-16.27) | 0.82 ± 0.03b** (22.38) | 0.88 ± 0.025b*** (51.72) |
| SA+EtOH | 250mg/kg | 18.1 ± 0.60b*** (-45.15) | 0.30 ± 0.012b*** (-30.23) | 0.95 ± 0.04b*** (41.79) | 0.94 ± 0.027b*** (62.06) |
| OMZ+EtOH | 20mg/kg | 15 ± 0.81b*** (-54.5) | 0.26 ± 0.14b*** (-39.53) | 1.02 ± 0.02b*** (52.23) | 0.99 ± 0.042b*** (70.68) |
Values are mean ± SE; EtOH: alcohol (50% v/v); OMZ: Omeprazole (20 mg/kg); SA: hydro-methanolic (20:80) extract of Sonneratia apetala leaves; t-test avs normal control, bvs EtOH control; *P<0.05,**P<0.01,***P<0.001; % change in parenthesis
Discussion
The annual global average of alcohol consumption is 6.4 L per person older than 1523. The World Health Organization estimates more than 2 billion people worldwide consume alcoholic beverages and nearly 76.3 million users having alcohol caused with problems24. It has been reported that consumption of alcohol disrupts the gastrointestinal barrier either by activation of inflammation-associated transcriptional factors, such as nuclear factor-kappaB or by up regulation of iNOS or overproduction of NO25. Experimental studies have also been confirmed that oxygen-generated free radicals and lipid peroxidation are involved in the pathogenesis of acute gastric lesions induced by alcohol17. It is therefore, well accepted hypothesis that oxidative stress plays an important role in the pathogenesis of gastric ulcers. Perhaps, there are also reports that antioxidants have played an important role in the protection of gastric mucosa against various necrotic agents10. Recent studies have shown that the antioxidant properties of herbs could be correlated with oxidative stress defence11,16. Plant polyphenols are antioxidants with redox properties, which allow them to act as reducing agents, hydrogen donators and singlet oxygen quenchers26. Previous studies point out that mangroves have rich source of novel compounds along with providing new source of many already known biologically active compounds with antioxidant properties13-15. In the present study, hydro-methanolic extract of Sonneratia apetala leaves (SA) exhibited all major phytoconstituents like phenolics, flavonoids, triterpinoids, steroids and tannins. It has been recognized that phenolics and flavonoids play a substantial role in the treatment of gastric ulcers27. Densitometric HPTLC fingerprint illustrated the presence of gallic acid, quercetin and coumarin in SA. There are also considerable pharmacological data to support that these three natural bioactive constituents have antioxidants, radical scavenging and antiulcer properties16.
Strong reducing capability and DPPH radical scavenging activity of SA demonstrate its antioxidant properties. Further, SA inhibited the formation of ABTS+ radicals. The discoloration of ABTS+ cation reflects the capacity of an antioxidant species to donate electrons or hydrogen atoms to deactivate harmful radicals14. SA also displayed nitric oxide and superoxide radical scavenging properties. iNOS and nitrotyrosine are considered to be key mediators in inflammation and oxidative stress pathways in relation with gastric ulcers25. Moreover, it has been considered that hydroxyl radical scavenging capacity is directly related to its antioxidant activity14. In the present study, SA also showed strong hydroxyl radical scavenging abilities. Therefore, it may be concluded that SA has the ability to scavenge free radicals. Other varieties of Sonneratia species and their different parts like bark, roots, fruits have also been reported for antioxidant potentialities, which corroborated the present findings2,4,6.
Reactive oxygen species (ROS), primarily super oxide anions, hydroxyl radicals and lipid peroxides are the harmful major contributors for gastric ulcer development, particularly in alcoholic states18,26. In this study, polyphenol rich SA showed potential preventive action in alcohol induced gastric mucosal damage in rats. Further, there are reports that unwarranted alcohol consumption created haemorrhagic injuries, extensive submucosal edema, mucosal frangibility and devastation to the epithelial cells of gastric tissue28. To scavenge ROS, gastric cells have several enzymatic and non-enzymatic intracellular antioxidants including catalase, superoxide dismutase, glutathione and sulfhydryl groups, but excessive generation of ROS augments lipid peroxidation and depletes these antioxidants26. But, in the present study, SA controls oxidative stress in gastric mucosal tissues as marked by reduced level of lipid peroxides and higher level of glutathione and catalase. Glutathione is one of the essential compounds for maintaining cell integrity because of its reducing properties and participation in the cell metabolism. Furthermore, catalse enzyme is a classical oxidative biomarker that exists mainly in peroxisomes of all aerobic cells and serves to protect the cells against damage from hydrogen peroxide10,28. Therefore, it assumed that hydro-methanolic extract of mangrove Sonneratia apetala leaves may lower the generation of ROS or nitric oxide radicals through its radical scavenging properties and thereby play a protective role in the prevention of mucosal haemorrhagic injuries by ethanol consumption.
Conclusion
From the above study it may conclude that Sonneratia apetala leaf has the capabilities to protect gastric ulcers caused by alcohol ingestion and that may be due to its strong radical scavenging abilities. Eventually, this study may lead to the development of effective compounds from Sonneratia apetala leaves for harmonizing therapeutic approaches to the treatment of complications in gastric ulcers, particularly in alcohol addicted population.