Highlights
Hyaluronic acid (HA), is a mucopolysaccharide present in almost all living beings, and has an essential role in cellular biology.
HA has advantages like biocompatibility, biodegradability, non-immunogenicity, and non-toxicity.
HA can recognize distinct receptors that are abnormally present in large numbers on the outer surface of cancerous tissues or cells.
HA is a promising biomaterial for the targeted delivery of anticancer agents alone or in combination with various other delivery systems like micelles, liposomes, hydrogels, and nanoparticles.
Introduction
Cancer is a disorder with uncontrolled cell growth and proliferation, which can sometimes be followed by metastasis. A neoplasm or tumor is a collection of cells that have grown out of control. They might form a lump or a mass but can also be dispersed1.
It is considered one of the fatal diseases, accounting for approximately 6 lakh deaths in 2020, and it was expected that this statistic would increase in millions in the next ten years. A coarser classification of histological based cancer is diagrammatically presented in Fig 1 3-6.
The establishment of successful cancer treatment has become the priority of researchers in the medical field. Chemotherapy is widely used as it involves drugs that regulate various mechanistic pathways like paclitaxel, docetaxel, doxorubicin, cisplatin, etc.7, However, chemotherapy is associated with systemic toxicity like nausea, fatigue, neutropenia, thrombocytopenia, etc.8.To overcome these limitations, the chemotherapeutics were coupled with materials or macromolecular ligands that would selectively bind with the expressed or overexpressed receptors macromolecules, on the outer surface or into the interior of diseased cancerous cells and hence facilitate the localization of drug at the target site reducing the undesirable effects as well as the dose of the chemotherapeutic agents for maintaining the required concentration at the desired target site.9 Various targeting materials were analyzed, and based on the biocompatibility and biodegradability properties, much attention was given to natural degradable polymers10.
Nanotechnology can precisely target anticancer agents to cancerous cells, lead to selective tumor degradation, and increase the therapeutic safety and effectiveness of all types of cancer therapy available.
Glycosaminoglycans (GAGs), an amino group-containing, non-complex polysaccharide, are made up of 2 distinct monosaccharide moieties attached in a repeated manner (uronic acid followed by an amino sugar (N-acetylglucosamine or N-acetylgalactosamine)). GAGs are found on the outer surface of cell membranes and in the interior site of cells. GAGs are essential in cell signalling, including cell growth, proliferation, cell adhesion enhancement, anticoagulation, and wound healing. They bind and regulate different proteins. Example of the most explicit Glycosaminoglycans includes: - keratan sulfate, chondroitin sulfate, heparan sulfate, heparin, and hyaluronic acid. Among all GAGs, the hyaluronic acid (HA) structure is the most straightforward because it does not have the sulfation of functional groups. This sulfation of the functional group occurs in the golgi apparatus for the other GAGs11 .
Methods
This narrative review is based on the literature searched in PubMed and the Elsevier database from January to May 2021 using the following keywords: “Hyaluronic acid”, “Hyaluronic acid in cancer therapy”, “Hyaluronic acid in cancer targeting”, “Hyaluronic acid in drug targeting”. Research published in the last five years was considered; however, no such timeline is followed in cross-referencing.
Results and Discussions
HA was first discovered as a non-complex linear-shaped biomolecule (polymer|) from bovine eyes in 1934.10 It is considered the vital portion of the extracellular matrix (ECM). It was further reported that HA plays a vital role in cell survival and maintains the structural wholeness, integrity, and stability of the cells and tissues.12-14 Cancer cells show abnormal accumulation of a large number of receptor proteins on the outer surface of a cell membrane that can recognize and bind to HA, as compared to normal cells, like the receptor for hyaluronic acid-mediated motility (RHAMM), the lymphatic vessel endocytic receptor (LYVE-1), and cell differentiation 44 (CD44) which supports the concept of HA-mediated selective tumor targeting.15
Chemical coupling of an anticancer drug with HA works by altering both the pharmacokinetics and pharmacodynamics of the drug. Coupling alters the solubility of the drug, altering release from formulation also its distribution in the body. This alteration in solubility can also increase the concentration at the site of action, which finally increases the pharmacological benefits of the drugs.16) Further study revealed that HA became a suitable candidate for chemical modification due to specific functional groups like hydroxyl, carboxyl, and N-acetyl, contributing to increased absorption, sustained release, improved drug targeting, etc. In this review, a brief discussion on using HA-based drug delivery systems to mitigate some previous problems associated with non-targeted delivery of toxic antineoplastic agents is also done.17
Source and structure of HA
HA was first recognized in 1934 by Meyer and Palmer and identified chemically in 1936.18
Sources of HA:
The sources of HA include tissues and fluids of vertebrates, ECM, and some species of bacteria. At the tissue level, HA is present in connective tissues like synovial medium, vitreous body, umbilical cord, and in integuments, cock’s combs, the dorsal fin of amphibians, brood spot of the birds, reproductive skin of monkeys, and the sex organs of fish, birds, and mammals. However, the HA content of the vitreous body of mammals and birds was found to get increased with age.19,20 The HA is also found in an intercellular matrix, golgi complex, synoviocytes (integumentary cells of the synovial membrane), vacuoles, and fibroblasts. In addition, HA is also regarded as a component of articular cartilage and skin. The bacteriological source includes hemolytic streptococci strains A and B.21
Structure of HA:
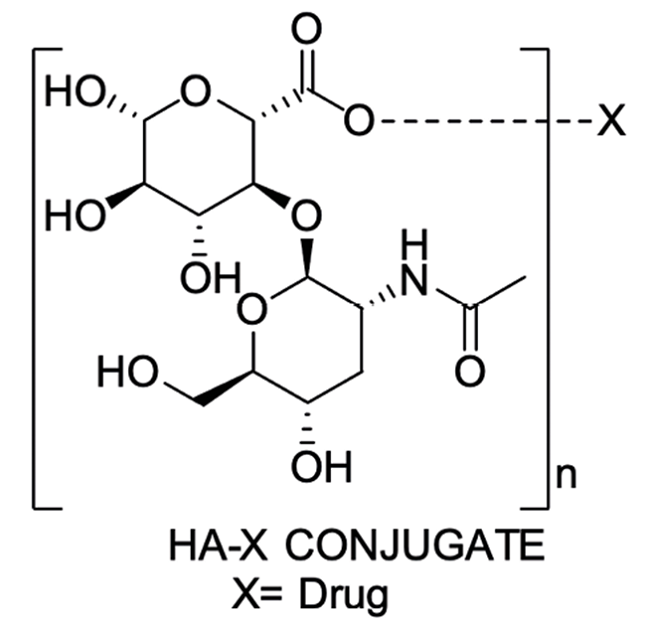
HA is an anionic linear mucopolysaccharide with molecular weight ranging from 4 - 2000 k Daltons.22 It is chemically made up of alternate two monosaccharide units of N- acetyl-D-glucosamine and D-glucuronic acid and linked by glucuronide bonds and glucosaminide bonds via respectively β-(1→3) and β-(1→4). In other words, HA is mentioned as a homopolymer of the hyaluronic acid, a disaccharide. The structure represented in Fig 2, suggests that HA does not possess intramolecular covalent bonds.23
Extraction and isolation of HA
The first isolation of HA took place in 1934 by Meyer. He isolated HA from the vitreous body of cattle, performing an aqueous-acetone extraction process.18 Selyanin et al. (2015) reported that Sewage also isolated HA in the same year but from human umbilical cords using an aqueous-chloroform extraction process. In 1936, HA was isolated from pig eyes’ vitreous bodies and bovine blood.24 For the first time, bacterial isolation was performed in 1937 by Kendall et al., including hemolytic streptococci sp. Up till now, many forms of isolation and extraction processes existed. However, all extraction methods deal with two common principles; first, the HA must undergo sequential breaking of its structures at each stage of localization, including homogenization, crushing, etc. Second, the HA must experience maximum contact with the extractants. It was found that the integrity of cellular structures of HA was broken down in disintegrators or by enzymatic action, or by using organic solvents and detergents, facilitating the isolation of HA from the complexes with other mucopolysaccharides and proteins21-23.
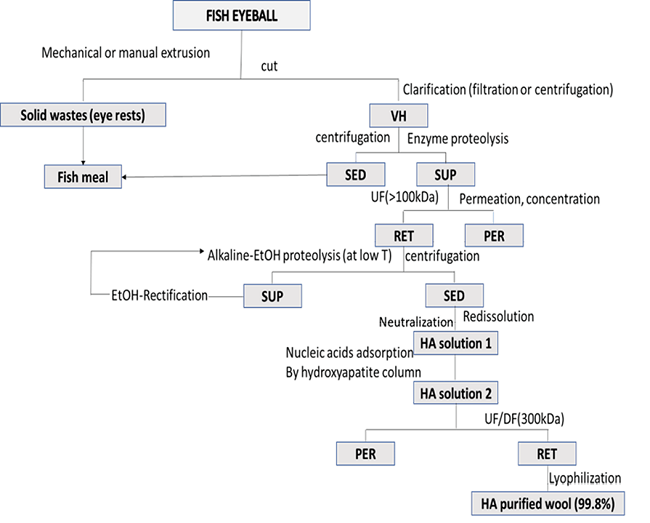
HA is extracted from tissues after the removal of traces of blood. Tissues are treated either with acetone or 95% ethanol in chloroform or with 90% acetic acid with sodium acetate. Washing is performed about 5-10 times till a clear solution is obtained. After washing, to avoid oxidative destruction, the washed tissues were either kept conserved in 95% v/v ethanol in a chamber having a temperature range of 4-22°C or subjected to a dried stream of acetone for an extended period. The obtained tissues were then crushed, dried, and swollen in 95% v/v ethanol or distilled water. Then the eye’s vitreous body and joints synovial fluid were purified from the treated tissue and were filtered. The extraction of HA from marine sources- fish eye is represented though a flowchart in Fig 3 23.
Extraction of HA from HA-protein complex occurs by (a) ultrafiltration, (b) Electro-dialysis, (c) Precipitating with cetylpyridinium, (d) Extraction with 90% phenol, sodium acetate, or chloroform-amyl alcohol mixture, (e) hydrolysis by papain enzyme, and (f) adsorption on activated charcoal. On the other hand, extraction of HA from HA- mucopolysaccharide complex occurs by (a) Electrophoresis, (b) Hydrolysis by enzymes, namely papain, trypsin, and pronase, or by 18 % HCl, (c) Precipitating with cetylpyridinium chloride, (d) Ion-exchange chromatography, and (e) Addition of salts of heavy metals.
The extracted HA is again subjected to reprecipitation with ethanol and cetylpyridinium chloride, followed by sterile acetone washing. The residue is then concentrated in a lyophilic dryer or vacuumed over phosphorus pentoxide. The purified and isolated HA obtained are stored at a temperature not more than - 18˚ C in dry form.23
Significance of HA in cancer therapy
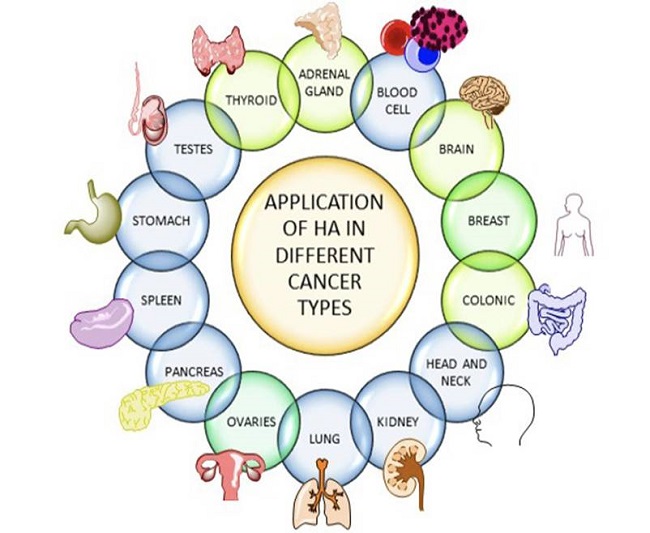
The tumor microenvironment is becoming increasingly important in determining neoplastic cell aggressiveness. The glycosaminoglycan hyaluronan is a major component of the tumor stroma and the surfaces of cancer cells, and its increase can significantly impact patient survival. HA is abnormally over-expressed by the vast number of cancerous cells, specifically tumor-developing cells. Since HA is implicated in various cancer-related malignancies (including enhanced cell proliferation, migration, invasion, angiogenesis, and chemo-resistance), blocking HA synthesis/signaling or reducing HA in tumor stroma might be a viable treatment approach for oncogenesis. The capacity of hyaluronan to interact with numerous signaling receptors, which frequently activate pro-angiogenic and pro-tumorigenic intracellular pathways, determines its activity. CD44 receptor that can recognize and bind with HA is overexpressed in pro-oncogenic and oncogenic cells and is associated with tumor progress, metastasis, and infiltration. The process is expressed in Fig 4. As a result, high levels of HA are identified in various types of cancer.
Role of HA in Cancer Chemotherapy
The presence of specific hydrophilic functional groups in HA, like hydroxyl, carboxyl, and acetamido groups, enabled intracellular hydrogen bonds, which provide polarity, hydrophilicity, and enhanced solubility of the molecule or drug. Such properties were used in the self-assembling of micelles and nanoparticles. The pKa of the carboxyl group is within 3-4, which allows HA to get dissociated in the physiological conditions. Hence, HA is a negatively charged anionic biomaterial that can react and combine with positively charged cations like sodium or potassium ions in the biological fluids to form respective hyaluronates. These properties support carriers’ improved loading capacity, controlled drug release, and increased active targeting12,25.
HA could be conjugated with the drug directly by a covalent bond to form HA-drug conjugates, which are not easily hydrolyzed or enzymolysis in the circulation, facilitating effective targeting and release of the drug. Such conjugates also enhance the drug’s solubility, distribution, and half-life, enhancing the osmotic retention effect and increasing drug concentration at a site or near the cancerous cells, showing better drug efficacy. Various cancers in which HA is used as carrier is presented in Fig 5.26
It was observed that these conjugates provide controlled release and targeted effect. The tolerable alterations in the chemical structure of HA could be done on the three principal functional groups, i.e., carboxyl, hydroxyl, and acetylamino groups, which are responsible for the activity. Cai et al. evolved a delivery system by chemically connecting the antiproliferative drug cisplatin to HA, increasing the cisplatin concentration in lymphatic vessels, reducing systemic toxicity, and preventing early metastasis.26 Similarly, Xin et al. (2010) developed HA-amino acid- paclitaxel conjugate by conjugating HA with paclitaxel via an amino acid-based cross-linker. It was observed that the presence of amino acids in the conjugates promoted the activity of esterase upon the conjugate, which enabled rapid release of paclitaxel, resulting in the early onset of action. Thus, it could be stated that HA-drug conjugation can switch or regulate the drug’s release profile and rate, influencing the therapeutic activity of cytotoxic antitumor drugs.27
Some instances show that some drug delivery carriers are surface-functionalized with HA, which aids in extending the circulation time of the carrier because of the biological origin of the HA, resulting in a sustained release pattern of the drug molecule from the drug delivery system28. In a very similar manner, HA- modified polylactic acid-glycolic acid copolymer nanoparticles (HCDs) were developed that showed ≈ 80% cumulative drug release in vitro in 14 days, indicating a sustained release profile, also showed an increased cellular uptake by cancer cells indicating an effective antitumor activity29.
Another concept in drug release and target effect via HA deals with its degradation by intracellular hyaluronidase or other enzymes. This concept became apparent from work conducted by Chen et al. (2018) where he prepared HA-coated Zr (IV)-based porphyrinic metal-organic framework (PZM) nanoparticle loaded with α-cyano- 4- hydroxycinnamate (CHC), monocarboxylate transporter 1 (MCT1) inhibitor molecule. HA facilitated the active targeting of the nanocarrier to the tumor cells. After reaching the target site, the system gets attacked by the hyaluronidase enzymes that degrade HA and release drugs to inhibit the activity of MCT130. Similarly, Gotov et al. (2018) prepared Docetaxel-loaded Gold nanoparticles (AuNPs), surface covered with HA via a linker molecule, cathepsin B-cleavable peptide (GFLGC). It was observed that HA improved the targeting of the AuNP in the diseased cancerous cells site, where the required high concentration of cathepsin B detached the GFLGC-linked HA via proteolysis, resulting in a sudden release of DTX and increased antineoplastic effect31. Hence, it could be inferred that HA is a versatile biomaterial that could be morphed either by modifying the functional groups within the structure or by surface functionalization via some linkers or polymers, resulting in modifying the release rate of the drugs as well as triggering active targeting in the tumor microenvironment.
Application of HA in cancer therapy
HA comprises distinct functional groups that help encapsulate active drugs or adsorption of macromolecules32. These properties make HA suitable for incorporating novel delivery systems and providing a platform for synergistic cancer treatment. HA-based delivery systems are selectively targeted to diseased cancerous cells based on the enhanced permeability and retention (EPR) effect and the reactions between HA and HA receptors, Namely RHAMM, LYVE-1, and CD44. Moreover, a negative surface charge on the HA-based delivery system allows its escape from the reticuloendothelial system (RES), improving the delivery of drugs in the diseased cancerous site33. For these reasons, HA-based drug delivery systems have been extensively explored. The HA-based delivery system for cancer treatment includes micelles, liposomes, hydrogel, polymersomes, and nanoparticles and are presented in Fig 6.
HA and its derivatives as carriers
HA and its chemically modified analogs can attach to specific receptors on the cell surface in various tissues, including the lymphatic arteries, kidneys, liver, and most cancerous tissues. The unique interaction of these systems with their specific receptors might be used to deliver drugs to locations and can be used as a system for targeted delivery of drug molecules. HA and other systems derived from chemical alteration in the HA have been widely used as vehicles or conveyors for specific delivery of giant biomolecules such as nucleic acids, proteins, peptides, and numerous drugs with large complex structures or structurally simple but having non-drug like characteristics.
For sustained-release therapy, HA can be utilized as a medication carrier. However, its poor steadiness is a negative factor in its utilization for gene vector delivery. In that case, it becomes essential to chemically alter HA to bring such changes that can further increase its steadiness or stability. HA is quickly destroyed in the human body by an enzyme called hyaluronidase. This degradation can be avoided by adding some foreign material to it. Compound, having a nitroxide-moiety preserve hyaluronic acid from degradation. An inhibitor of hyaluronidase also prevents hyaluronic acid breakdown by decreasing the activity.
Incorporating foreign material to protect HA-based systems against degradation allows us to utilize its drug carrier system in cancer treatment therapy. The HA receptor CD44 is now reported to be overexpressed in numerous tumor cells and expressed significantly less on the outer surface of the cell membrane of neuronal cells, hematopoietic cells, and epithelial cells. The drug chemical moiety is covalently joined or connected to the carrier because of the carrier’s specific drug delivery mechanism. The medication is delivered to the desired diseased neoplastic cells, tissues, or organs via the carrier’s characteristic of specific and targeted recognition phenomenon inside the human beings.
Drug distribution into the body is dependent and characterized by the carrier’s specific properties. At the moment, tumor-targeted treatment has used chiefly the EPR principle. The most widely used mode of active targeting includes the specific interaction between the carrier system and one of the biological components inside the body mainly located nearly or into the desired targeted site in colossal amount compared to its distribution or occurrence in other locations in the body. These biological components may be the specific sequence of polypeptides, antibodies, or the receptors’ protein molecules. HA can specifically interact and combine with receptors and protein molecules and therefore plays a vital role in various biological processes, such as angiogenesis, regulation of cell division processes, repairing damaged or injured tissues, and formation of human tissues. HA and its chemically altered modified derivatives as drug delivery or conveyor system for the effective treatment of various diseases via several medicaments such as anesthetics, anti-inflammatory, and antitumors.
A specific example of HA-based drug delivery is 5-fluorouracil (5-FU). The 5-FU is a first choice or line drug to prevent and mitigate various cancers, including breast, neck, head, and colorectal. 5-FU has high toxicity for skin, GIT, heart, nervous system, and blood and blood-forming components; toxicity in skin includes mainly sensitization and dermatological reactions. 5-FU is a fluoropyrimidine that is still widely considered the gold standard for various malignancies, from various types of cancer to immunological diseases. Due to its high toxic effects on various organs, targeted delivery is needed to limit its distribution in the body to a specific desired diseased area. 5-FU-loaded HA composite microparticles have a favorable drug release profile, making them a potential delivery method for the controlled release of chemotherapeutic medicines in cancer treatment.
HA-based micelles for treatment of cancer
The concept of drug delivery by micelles formation has the drawback of balance between hydrophilicity and lipophilicity of drug molecule, concentrated but lesser amount of drug-carrying capacity, low stability and unrequired predestination release of the drug, etc. These problems give rise to a new and more advanced drug delivery system: the prodrug micelles system. Some studies show that the micellar prodrugs can be effectively used to develop such a system based on HA for the treatment of the various types of neoplasms and malignancies34.
Micelles are colloidal dispersions composed of amphiphilic moieties ranging from 20-100nm. Self-assembled polymeric micelles are conjugated with HA to form an amphiphilic nanocarrier. The hydrophobic core of the HA-based micelles allows encapsulation of hydrophobic drugs by chemical, physical and electrostatic interactions. In contrast, the hydrophilic surface allows increased system circulation to accumulate in cancer cells via the EPR effect. Also, coating HA with positively charged nanocarrier offers enhanced antitumor activity34,35.
As for almost 90% of drugs, the principal transfer route for the doxorubicin is passive diffusion from the lipid bilayered cell membrane and nuclear membrane, and it is concentrated within the cell nucleus and causes cells to undergo apoptosis36. Lammers et al. (2009) found that doxorubicin (DOX) prodrug micelles based on poly (ethylene glycol)-b-poly(N-(2-hydroxypropyl) methacrylamide-lactate) and DOX-MA (DOX methacrylamide) showed more pronounces effect for the cancer cells progression and spreading. Drug targeting from these micelles also showed a higher survival rate of neoplastic cells in B16F10 melanoma (mice model). The DOX micelles were more therapeutically effective in the cancer model (B16F10 melanoma) than the drug solution. It was also found that the subjects’ encounters with the DOX micelles were a long time of survival compared to those who only took the DOX drug solution. One more benefit of micelles is that they show no adverse effects observed with the drug solutions at the same dose with the same route.37
Yin and his group (2015) prepared micelles of conjugated HA- Paclitaxel (PTX), having a disulfide bond (HA-ss-PTX) that can be broken down by redox reactions for the treatment of breast adenocarcinoma35.
Lee et al. (2009) prepared DOX-loaded HA- PLGA micelles to improve the cellular uptake of DOX in tumor sites and established its cytotoxicity in human colon cancer cells (HCT-116)36.
Li et al (2012) developed PTX-packed self-assembled micelles conjugated with hyaluronic acid-deoxycholic acid; these conjugates are redox-sensitive and are used to determine intracellular targetability in adenocarcinoma of human breast cells38.
Choi et al., (2012) prepared a micelle for the PEG-HA conjugation system that enclosed the Irinotecan (IRT) within the lipophilic cores of the micelle for the treatment of colon cancer39.
Qui and his associates (2014) formulated a pH-responsive-based micellar delivery system by conjugating HA with lipophilic poly(L-histidine) (PHis) to determine the targeting property of the carrier system in the breast cancer cells (MCF-7)40.
Thomas et al. (2014) prepared paclitaxel (PTX) packed self-assembling, biodegradable micelles conjugated with HA to target HA-responsive CD44 receptors for cancer treatment into an artificial cell line neck and head cancer cells (SCC7)41.
Shin and his associates (2017) developed a micelles system that selectively responds to the MMP9 (matrix metalloproteinase 9). This system is comprised of OVA peptide and PEGylated HA for the murine cervical cancer cells (TC-1) model42.
Mao et al. (2019) prepared a prodrug micelle that selectively responds to GSH. It comprises DOX, HA, and amphiphilic ferrocenium-tetradecyl (Fe-C14) to treat prostate cancer (PC3)43.
HA-based liposomes for cancer therapy
Liposomes are amphiphilic and made up of a phospholipid bilayer, having a diameter ranging from 50-100nm. In liposomes, the hydrophobic core aids in the entrapment of hydrophobic drugs, whereas the hydrophilic part aids in transporting hydrophilic drugs in the aqueous surroundings. Nowadays, liposomal nanoparticle-based drug delivery systems can be taken as the advances in the applicability of nanotechnologies for advancing cancer therapy. These day-by-day growing technologies have many benefits over widely prevalent conventional preparations of liposomes for cancer mitigation. The advantages are lesser toxicity, control over the time and rate of release pattern of API constituent from the preparation, increased physical stability inside the body, and biocompatibility.43
Liposomes were also approved for drug delivery due to their longer circulation time into the blood, enhanced penetration ability, and controlled diffusion properties. However, liposomes exhibit limitations like poor in vivo stability and drug loading maintenance15.
Taetz et al. (2009) developed positively charged HA - liposomes modified with dioleoyl phosphatidylethanolamine / 1,2-dioleoyl-3- trimethyl ammonium propane (DOPE/ DOTAP) for targeting anti-telomerase siRNAs into the cells positive for CD44 for the mitigation of lung cancer44.
Wojcicki et al., (2012) formulated HA-DOPE liposomes for transfecting the lung cancer cells.45
Rivkin et al. (2010) formulated liposomal preparation containing 1,2-Dilauroyl-sn-Glycero-3-Glycerol and 1,2-Dilauroyl-sn-Glycero-3- Phospho-ethanolamine, encapsulating paclitaxel (PTX) for the treatment of colon cancer (CT-26), which was further coated with HA by a covalent bond46.
Similar advantages were observed when Yang et al. (2013) prepared cationic paclitaxel phosphatidylcholine liposomes, electrostatically conjugated with HA for targeting colon cancer (HCT 116) and Melanoma (B16).47
Peer and Margalit (2004) also prepared liposomes coated with HA-containing the lipids like cholesterol, phosphatidylethanolamine, and phosphatidylcholine for encapsulating mitomycin C in treating lung cancer.48
HA-based hydrogel for cancer therapy
Hydrogels are hydrated 3D polymeric networks capable of absorbing 10 - 20% water. They are highly porous in structure which helps them deliver drugs in a predetermined mode that, in turn, depends upon the drug-carrying capacity within the gel matrix.
Hydrogels have recently gained so much attraction from researchers as polymeric systems for the evolution of biomaterial, owing to their sensitivity and responsiveness to various stimuli and their easily manipulated characteristics. These polymeric systems are very similar to natural soft tissues because of the similarity in their flexibility, softness, and a considerable amount of water carrying capacity. So, these gel systems can become a suitable alternate indisputably for the ECM.
Unique properties of these complicated 3D polymeric hydrogels, researchers have gained notable development in tumor diagnosis, reconstruction of tumor models, and other relative therapies46.
However, the hydrogel has some demerits, including poor mechanical strength, and is prone to degradation. Hence, structural alternations like chemical modifications and covalent cross-linking were performed to conjugate HA with hydrogel for a logical and effective drug delivery system15.
Bajaj et al. (2012) prepared PTX-loaded HA-hydrogel to mitigate ovarian carcinoma49.
Jhan et al. (2015) formulated HA-Dox nano-complex, later coated with Pluronic F127 to form a thermosensitive hydrogel to treat colon cancer (C26)50.
Cho and his associates (2015) developed platinum nanoparticles cross-linked with HA-coated hydrogel (PtNP/HA gel) against ovarian cancer51.
Fu et al. (2015) developed dual stimuli-responsive DOX-loaded hydrogel and conjugated it with thiolate HA to treat nasopharyngeal carcinoma (CNE2)52.
Ueda and his associates (2016) incorporated IFNα into HA-coated hydrogel to mitigate metastatic cancer of renal cells53.
Shin et al. (2017) prepared HA-conjugated vaccine adjuvants comprising hydrophobic monophosphoryl lipid, QS21, and imiquimod (R837) against ovarian cancer.54
Yang G et al. (2017) developed methacrylate-linked HA hydrogel loaded with DOX to mitigate liver cancer, facilitating enhanced targeting of the anticancer agents at the tumor site55.
HA-based nanoparticles for cancer treatment
Nanoparticles (NPs) like gold NPs (AuNP), silica-based NPs (SiNPs), magnetic NPs, quantum dots, ceramic NPs, and carbon-based NPs, are widely used for cancer treatment. However, nanoparticles exhibit certain drawbacks like less specificity and increased cytotoxicity. Thus, the surface of the NPs was modified with biopolymers for functionalization, resulting in the emergence of cancer theragnostic. Hence, surface modification of NPs with HA provides a system with much biocompatibility and specific cell targeting ability15.
Li et al. (2017) synthesized HA-coated mesoporous silica NPs (MSN NPs), loaded with PTX for targeting MCF-7 breast cancer cells56.
Fu et al. (2017) synthesized HA-DOX NPs, which showed an enhanced water solubility profile of DOX and increased accumulation in liver cancer cells (HepG2)57.
Lee et al. (2014) prepared calcium phosphate NPs (CAP-NPs) and conjugated them with DOPA and HA to efficiently deliver siRNA to the solid tumor site58.
Wang et al. (2014) synthesized HA conjugated quantum dots (HA-QD) that function as CD44+ overexpressed tumor cell-targeted imaging probes. The synthesized HA- QDs exhibited improved fluorescence stability in biological fluids, which help target CD44+ breast cancer cells without causing cellular cytotoxicity59.
Yang et al. (2017) also prepared QDs, coated with magnetic Prussian blue conjugates and HA (HA-PB-QD), functioning as cancer cell theranostics. It was observed that HA-PB-QD was significantly taken up by the CD44+ cervical carcinoma cells (HeLa cells) due to the coexistence of HA and magnetic natured PB60.
Yun et al. (2004) prepared HA-cross-linked NP encapsulating β-gal plasmid to deliver DNA in cancer therapy61.
Liu et al. (2015) synthesized 5 FU-loaded HA-SiNPs to increase the therapeutic activity of 5-FU in colon cancer cells. HA-SiNPs provided sustained release of 5 FU and inhibited its rapid clearance. The clearance was delayed because of the surface modification on the NP’s surface, which involved an EDC reaction between the amine group of SiNPs and the carboxylic group of HA, which further facilitated an enhanced cellular uptake SiNPs by the cancer cells62.
Cho et al. (2011) prepared DOX-loaded ceramide-HA (HA-CE) conjugated NPs for targeted delivery in breast cancer cells (U87-MG and MCF-7). They developed similar DOX-loaded HA-CE NPs, to treat CD44+ squamous cell carcinoma (SCC7)63.
Wang et al., (2016) developed PTX-loaded HA-NLCs for improved therapeutic activity against ovarian cancer64.
Gene Conjugation
Genetic material can be delivered from 2 different modes using non-viral and viral vectors. Non-viral vectors have a lower capacity and efficiency of transfection (gene). Adeno-associated viruses, lentiviruses, and retroviruses are examples of viral vectors and are more effective for inserting a new gene into a host. Despite the high capacity and efficiency, the viral vectors are not commonly used because they infect the host and cause toxic and immunological responses that are undesirable or, in some cases, not tolerable. These limitations avoid using viral vectors and turn the choice toward the non-viral vectors. Non-viral vectors also provide the advantages of conjugation for targeted delivery and have the capacity to load a high amount of genes per unit vector65.
Oligonucleotides surface coated NPs have recently gained attraction for their usefulness in gene therapy. These Oligonucleotides surface coated NPs structures exhibit high cell uptake and more effective gene transfection without utilizing other transfection agents65.
Above all, these Oligonucleotides surface coated NPs exhibit enormously efficient gene-regulating abilities, lesser toxicity, least immune responses, and resistance to destruction by the enzyme nuclease66-68.
Oligonucleotides surface-coated NPs may be applicable for gene therapy and gene delivery.
Park et al., 2013- HA−siRNA/LPEI complex was prepared in the hope of its applicability in gene silencing; they conjugated siRNA to HA (polyanionic and flexible). Finally, the targeted systemic introduction of the HA−siApoB/LPEI system effectively down-regulated ApoB mRNA levels in the liver carcinoma. The targeted specific HA−siRNA complex system may potentially be applicable in mitigating liver cancers and other malignancies69.
Yin et al. (2015) suggested that ‘Cyclodextrins have mainly been explored to enhance membrane permeability, improve bioavailability, and mitigate the toxic effects of substances. Activation of CD44 increased its downstream processes, leading to cancer cell proliferation, metastasis and progression. Due to the selective interaction between CD44 receptors and HA can be used in cancer treatment (CD44 positive). This system is based on HA-conjugated β-CD-OEI-HA polymer β-CD star polymer with multiple oligoethylenimine (OEI) arms). This polymer showed non-toxicity and has more capacity to incorporate pDNA and selectivity for efficiently introducing a gene in CD44-positive cancer cell lines (MDA-MB-231 breast cancer cells). Finally, the increased survival of cancerous cells and effective cell division mitigation demonstrates its high potential for gene delivery in various malignancies, including cancers that are CD44 positive70.
The various HA-based drug deliveries developed in the last decade to improve target ability and increase the therapeutic activity of various anticancer drugs in cancer therapy have been summarized in Table 1.
Table 1: The various HA-based drug delivery systems developed in the last decad.
| S. No. | Cancer | Therapeutic candidate | Observations | Refe. |
|---|---|---|---|---|
| HA-based Drug (direct) conjugation | ||||
| 1 | Human colon cancer | Paclitaxel-HA | Significantly fewer toxic effects, Provide more excellent selectivity | (71) |
| 2 | Human breast cell carcinoma | Doxorubicin-HA | The high anticancer effect, less cardiac toxicity, Greater inhibition effect, long-lasting release | (72) |
| 3 | Murine cervical cell carcinoma | HA-ovalbumin | Fewer side effects, targeted delivery, less toxicity | (73) |
| 4 | Mammary carcinoma | R848 (Immuno), HA-doxorubicin (chemo) | The high anticancer effect, less cardiac toxicity, Greater inhibition effect, long-lasting release | (74) |
| HA based micelles | ||||
| 5 | Squamous cell cancer | 5B-cholanic acid-HA, paclitaxel | Increased accumulation, Effective intracellular uptake, High drug-carrying capacity for lipophilic drug | (40) |
| 6 | Breast cancer | Deoxycholic acid-HA, paclitaxel | Low systemic toxicity, Increased accumulation, Effective intracellular uptake, High drug-carrying capacity for lipophilic drug | (38) |
| 7 | Human colorectal cancer | 5B-cholanic acid-HA-PEG, irinotecan | Effective intracellular uptake, High drug-carrying capacity for lipophilic drugs, Greater inhibition effect, long-lasting release | (39) |
| 8 | Murine cervical cell carcinoma | PEG-pep-HA, ovalbumin | Increased accumulation, Effective intracellular uptake, High drug-carrying capacity for lipophilic drugs, very fewer toxic effects, Provide more excellent selectivity | (42) |
| 9 | Human lung cell cancer | Hypocrellin B (PDT), paclitaxel (chemo), HA-ceramide | Effective intracellular uptake, High drug-carrying capacity for lipophilic drugs, Greater inhibition effect, long-lasting release | (75) |
| 10 | Breast adenocarcinoma | Paclitaxel (PTX) | Micelles provided burst release of PTX at the tumor site, followed by enhanced cytotoxicity and apoptosis. | (35) |
| 11 | Colon cancer | Doxorubicin (DOX) | DOX-HA-PLGA micelles showed enhanced cellular uptake via receptor-mediated endocytosis and increased cytotoxicity with IC50 of 0.67μg/mL, as compared to free DOX(IC50=3.48 μg/mL) | (36) |
| 12 | Breast adenocarcinoma | PTX | The HA-based micelle showed rapid release of PTX from the system. Also, the HA micelles showed enhanced cellular uptake along with increased cytotoxicity (IC50 = 25.6 ng/mL), as compared to free PTX (IC50 = 51.7 ng/mL) | (38) |
| 13 | Colon Cancer | Irinotecan (IRT) | HA-based micelle showed enhanced tumor targeting with reduced systemic side effects. | (39) |
| 14 | Breast cancer | DOX | Micelle provided pH-dependent DOX release, followed by rapid cellular uptake via CD44+ receptor-mediated endocytosis. | (40) |
| 15 | Head and neck cancer | PTX | PTX-HA micelles showed enhanced cellular uptake and cytotoxicity for the 1-100 μg/mL concentration. | (41) |
| 16 | Breast cancer | PTX | Micelle showed high drug loading capacity, lysosomal pH mediated rapid release of PTX, and efficient cytotoxicity compared to free PTX. | (76) |
| 17 | Cervical cancer | Ovalbumin (OVA) | The micellar system showed enhanced cellular uptake, accompanied by apoptosis. | (42) |
| 18 | Prostate cancer | DOX | The micellar system showed rapid dissociation of micelles, resulting in rapid release of DOX, followed by enhanced Cytotoxicity, as compared to free DOX. | (43) |
| HA based polymersome | ||||
| 19 | human breast cell cancer | DSPE-PEG-HA, doxorubicin | More selective, High anticancer efficacy, the pH-dependent release of drug | (77) |
| 20 | human lung small cell cancer | DSPE-PEG-HA, pDNA | Longer retention, the pH-dependent release of drug | (78) |
| 21 | Melanoma | DSPE-PEG-HA, siRNA (for TGF-β) | High drug-carrying capacity for lipophilic drug, Greater inhibition effect, long-lasting release, more selective, High anticancer efficacy, the pH-dependent release of drug | (79) |
| 22 | mammary cell cancer | Marimastat (TME), HA-paclitaxel (chemo) | More selective, High anticancer efficacy, the pH-dependent release of drug | (80) |
| HA Based Hydrogel complex | ||||
| 23 | human ovarian cell carcinoma | paclitaxel | Longer retention, highly viscous, high and longer localized concentration of the drug, suitable for chronic treatment | (49) |
| 24 | human renal cell cancer | HA-tyramine, IFN-α, sorafenib | Orally, the long-acting, large amount of drug can be loaded, Longer retention, the highly viscous, high, and longer localized concentration of the drug, suitable for chronic treatment | (53) |
| 25 | Lymphocyte cell cancer | Monophosphoryl lipid, QS21, R837 + HA | Low systemic toxicity, Increased accumulation, Effective intracellular uptake, High drug-carrying capacity for lipophilic drug, Longer retention, the highly viscous, high and longer localized concentration of the drug, suitable for chronic treatment | (54) |
| 26 | human breast cell carcinoma | Doxorubicin (chemo), mRNA (gene), HA-chitosan | Longer retention, highly viscous, high and longer localized concentration of the drug, suitable for chronic treatment | (81) |
| 27 | human lung cell cancer | Graphene (PTT), doxorubicin (chemo), HA-disulfide | Longer retention, highly viscous, high and longer localized concentration of the drug, suitable for chronic treatment | (82) |
| 28 | Ovarian cancer | PTX | PTX-HA-hydrogel showed enhanced retention in the intraperitoneal cavity, resulting in increased accumulation of PTX at tumor site, followed by reduced tumor volume. | (47) |
| 29 | Colon cancer | DOX | HA-coated hydrogel significantly inhibited tumor growth, and the presence of HA facilitated lymphatic uptake. | (28) |
| 30 | Ovarian cancer | Platinum (Pt) | PtNP/HA gel showed prolonged retention in the peritoneal cavity, where PtNP was released slowly and was selectively targeted to CD44+ cancer cells. | (51) |
| 31 | Nasopharyngeal carcinoma | DOX | HA-DOX hydrogel offered sustain release profile of DOX. The hydrogel also showed significant tumor growth inhibition compared to free DOX. | (50) |
| 32 | Renal cell carcinoma | Interferon-α (IFN-α) | HA-hydrogel significantly inhibited tumor growth, as well as suppressed tumor proliferation, resulting in apoptosis, and anti-angiogenesis. | (53) |
| 33 | Ovarian cancer | Imiquimod (R837) | HA-Hydrogel effectively suppressed the tumor proliferation, resulting in apoptosis. | (52) |
| 34 | Liver cancer | DOX | DOX-loaded HA hydrogel facilitated enhanced targeting at the tumor site via CD44+ receptor-mediated endocytosis, with reduced systemic toxicity. | (57) |
| HA Based Nanoparticles | ||||
| 35 | human breast carcinoma | Gold nanoparticle (PTT), doxorubicin (chemo), HA-dopamine | Decreased toxicity, increased water solubility, prolonged release, increased acceptability | (83) |
| 36 | Breast cancer | PTX | HA-PTX-loaded MSN NPs showed enhanced accumulation via receptor-mediated endocytosis, followed by enhanced tumor cell suppression and limited toxicity compared to free PTX. | (56) |
| 37 | Liver cancer | DOX | HA-DOX NPs showed increased water solubility and increased tumor cell accumulation. The release of DOX from NPs was pH-dependent. | (57) |
| 38 | Solid tumors | siRNA | HA-NPs protect the siRNA from enzymatic degradation, facilitating siRNA targeting at the solid tumor site. | (58) |
| 39 | Breast cancer | Quantum dots (QD) | HA-QD was used as an imaging probe to help target the CD44+ breast cancer cells without causing systemic toxicity. | (59) |
| 40 | Cervical carcinoma | Prussian blue (PB) - QD | HA-PB-QD functions as cancer cell theranostic. They are significantly taken by CD44+ cancer cells, showing an 89.95% reduction in tumor volume. | (60) |
| 41 | Cancer | β- gal plasmid | HA-NP showed controlled release of DNA in the tumor sites with fast transfection (60 days). | (61) |
| 42 | Colon cancer | 5- Fluorouracil (5-FU) | HA-5FU-SiNPs showed sustained release of 5 FU accompanied by delayed renal clearance. | (62) |
| 43 | Breast cancer | DOX | HA-CE-DOX-NPs showed enhanced cellular uptake, and increased cytotoxicity (IC50 =200.94 ng/mL), as compared to free DOX (IC50 = 966. 43 ng/mL). | (63) |
| 44 | Squamous cell carcinoma | DOX | NPs showed enhanced receptor-mediated cellular uptake, improved cytotoxicity profile, and reduced tumor volume (19%) as compared to free DOX (48.7%). | (64) |
| 45 | Ovarian cancer | PTX | HA-PTX-NLC showed efficient inhibition of cell viability in vivo and reduced tumor volume compared to free PTX. | (65) |
| HA Based Nanoparticle (surface modification) | ||||
| 47 | Human breast cell cancer | Si nanoparticle, paclitaxel | Strongly oppose cancer, few toxic and side effects, High anticancer effect, more uptake in the tissues, more selective | (56) |
| 48 | Liver cell cancer | SPION-HA, doxorubicin | Specific and selective accumulation, Biologically recognizable, High solubility in the water | (57) |
| 49 | Colorectal cancer | Calcium phosphate-HA, siRNA | Strongly oppose cancer, few toxic and side effects, High anticancer effect, more uptake in the tissues, more selective | (58) |
| HA based Complex (electric interaction) | ||||
| 50 | WBC cell subtype CDD4 cell cancer | Polyethyleneimine-HA, siRNA | Uptake via Receptor More inhibition potential | (84) |
| 51 | Kidney cell cancer, melanoma | Polyethyleneimine-HA, siRNA | More uptake, more inhibition, receptor-mediated recognition, specific for the target | (85) |
| HA based Gene conjugation | ||||
| 52 | HCT-116 (human colon cancer | siRNA-HA | Less systemic availability, more uptake, more inhibition, receptor-mediated recognition, specific for the target | (86) |
| HA based Liposomal drug delivery system | ||||
| 53 | Lung cancer | siRNA | HA-based liposomes showed enhanced targeting via CD44+ receptor-mediated endocytosis. Further, the presence of HA protected siRNA from RNaseV1. | (44) |
| 54 | Lung cancer | DNA | HA-DOPE liposomes showed significantly enhanced transfection as compared to Lipofectamine. | (78) |
| 55 | Colon cancer | PTX | HA-liposomes showed increased accumulation of PTX at the tumor site with less distribution in the spleen and liver. Also, HA-liposomes showed reduced tumor volumes compared to free PTX. | (39) |
| 56 | Colon cancer and melanoma | PTX | HA-liposomes showed increased antitumor activity and reduced tumor volume compared to free PTX and non-coated liposomes. | (62) |
| 57 | Lung cancer | Mitomycin C | HA-coated liposomes showed enhanced drug efficacy, increased accumulation of mitomycin C at the tumor site, and reduced tumor volume with no systemic side effects. | (48) |
Conclusion
The developments in nanotechnology paved a new and promising way in cancer, including prevention and therapeutics. Most instantly, HA is employed as a carrier for antineoplastic drugs due to its targeting ability and susceptibility to degrade via hyaluronidase enzyme inside the body. The research concluded that targeting cancer cells responds to overexpression of HA receptors, majorly CD44. Similarly, HA-based nanoparticles actively target and enter the cancer cells via CD44+ receptor-mediated endocytosis. The researchers developed various HA-based nanocarriers; the most prominent are micelles, liposomes, hydrogels, nanoparticles, and others. These HA-based nanocarriers can effectively deliver the anticancer drugs at the tumor site via passive targeting (by EPR) or active targeting (via receptor-mediated phenomena). These HA-based nanocarriers provide advantages over other delivery systems, including higher biocompatibility, prolonged circulation time, enhanced cellular uptake, and improved stability. They have significant PDT, PTT, MRI, and fluorescence imaging applications in the theragnostic field. Expectations towards elaborative strategies in biomedical applications of HA for the efficient treatment of cancer in the future are high.