My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
REC: Interventional Cardiology
On-line version ISSN 2604-7276Print version ISSN 2604-7306
REC Interv Cardiol ES vol.5 n.4 Madrid Oct./Dec. 2023 Epub Mar 04, 2024
https://dx.doi.org/10.24875/recic.m23000386
Original articles
Left atrial appendage occlusion vs oral anticoagulants in AF and coronary stenting. The DESAFIO registry
aSección de Cardiología Intervencionista, Servicio de Cardiología, Hospital Universitario de Badajoz, Badajoz, Spain
bSección de Cardiología Intervencionista, Servicio de Cardiología, Hospital Universitario de Salamanca, Instituto de Investigación Biomédica de Salamanca (IBSAL), Salamanca, Spain
cCentro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV), Spain
dSección de Cardiología Intervencionista, Servicio de Cardiología, Instituto Cardiovascular, Hospital Clínico San Carlos Madrid, Spain
eSección de Cardiología Intervencionista, Servicio de Cardiología, Hospital Clínico de Barcelona, Barcelona, Spain
fSección de Cardiología Intervencionista, Servicio de Cardiología, Hospital Álvaro Cunqueiro, Vigo, Pontevedra, Spain
gSección de Cardiología Intervencionista, Servicio de Cardiología, Hospital Universitario de la Santa Creu i Sant Pau, Barcelona, Spain
hSección de Cardiología Intervencionista, Servicio de Cardiología, Hospital Universitario de La Paz, IdiPAZ, Madrid, Spain
iSección de Cardiología Intervencionista, Servicio de Cardiología, Hospital Universitario de Puerta de Hierro, Majadahonda, Madrid, Spain
jSección de Cardiología Intervencionista, Servicio de Cardiología, Hospital Clínico Universitario de Valladolid, Valladolid, Spain
kSección de Cardiología Intervencionista, Servicio de Cardiología, Hospital Universitario de León, León, Spain
Introduction and objectives:
The treatment of patients with non-valvular atrial fibrillation (NVAF) who need coronary stenting is challenging. The objective of the study was to determine whether left atrial appendage occlusion (LAAO) could be a feasible option and benefit these patients. To this end, we studied the impact of LAAO plus antiplatelet drugs vs oral anticoagulants (OAC) (including direct OAC) plus antiplatelet drugs in these patients' long-term outcomes.
Methods:
The results of 207 consecutive patients with NVAF who underwent coronary stenting were analyzed. A total of 146 patients were treated with OAC (75 with acenocoumarol, 71 with direct OAC) while 61 underwent LAAO. The median follow-up was 35 months. Patients also received antiplatelet therapy as prescribed by their cardiologist. The study received the proper ethical oversight.
Results:
Age (mean 75.7 years), and the past medical history of stroke were similar in both groups. However, the LAAO group had more unfavorable characteristics (history of coronary artery disease [CHA2DS2-VASc], and significant bleeding [BARC ≥ 2] and HAS-BLED). The occurrence of major adverse events (death, stroke/transient ischemic events, major bleeding) and major cardiovascular events (cardiac death, stroke/transient ischemic attack, and myocardial infarction) were significantly higher in the OAC group compared to the LAAO group: 19.75% vs 9.06% (HR, 2.18; P = .008) and 6.37% vs 1.91% (HR, 3.34; P = .037), respectively.
Conclusions:
In patients with NVAF undergoing coronary stenting, LAAO plus antiplatelet therapy produced better long-term outcomes compared to treatment with OAC plus antiplatelet therapy despite the unfavorable baseline characteristics of the LAAO group.
Keywords: Stents; Atrial appendage; Atrial fibrillation; Anticoagulants
INTRODUCTION
Patients with atrial fibrillation (AF) who undergo percutaneous coronary intervention (PCI) with coronary stenting are a subset in whom antithrombotic treatment is particularly complex. In this challenging scenario, anticoagulant therapy is the treatment of choice for stroke prevention while dual antiplatelet therapy (DAPT) is the treatment of choice for preventing stent thrombosis and future coronary events. Combining both drug types, however, increases the risk of bleeding.1
This problem will only rise in prominence since the rate of AF and coronary artery disease increases with age and elderly patient populations continue to grow.2
The rate of coronary artery disease in patients with non-valvular atrial fibrillation (NVAF) is as high as 30%. As a matter of fact, nearly 20% of the patients undergo coronary revascularization, especially PCI.3 Furthermore, approximately 6% to 8% of the patients admitted due to acute coronary syndrome (ACS) have AF.4 The higher mortality rates seen in these patients (between 2- and 3-fold at 5 years) may also be related, among other factors, to the need for combined anticoagulant and antiplatelet (AP) drug therapies and the high rate of associated bleeding events.5 In fact, the effect post-discharge bleeding has on all-cause mortality at 2 years has been associated with higher crude all-cause mortality rates (13.0% vs 3.2%; P < .0001; hazard ratio [HR], 5.03; P < .0001) with an effect size greater than that of myocardial infarction after discharge (HR, 1.92; P < .009).6
Left atrial appendage occlusion (LAAO) has been shown to reduce bleeding compared to oral anticoagulants (OAC) in patients with a high risk of bleeding.7-9 This strategy may also allow patients to continue DAPT possibly reducing ischemic events with fewer bleeding events compared to the anticoagulant-AP therapy combo.
Our objective was to determine whether LAAO could be a feasible option and benefit these patients. To this end, we studied the impact of LAAO plus AP versus OAC (including direct OAC [DOAC]) plus AP in these patients' long-term outcomes regarding mortality prevention, ischemic and hemorrhagic events (figure 1).

Figure 1. Central illustration. AMI, acute myocardial infarction; CI, confidence interval; DES, drug-eluting stent; HR, hazard ratio; LAAO, left atrial appendage occlusion; MACE, major adverse cardiovascular events; MAE, major adverse events; NVAF, non-ventricular atrial fibrillation; OAC, oral anticoagulants; TIA, transient ischemic attack.
METHODS
This was a multicenter, observational study of 2 historical cohorts of patients. Back in 2021, 11 Spanish centers were asked to participate in a registry of patients who had received LAAO with an indication for OAC withdrawal in the presence of a high risk of bleeding when this indication coexisted with that of DAPT following intracoronary stenting. Inclusion went on through March 2021. Patients treated with LAAO were compared to a consecutive series of patients with an indication for anticoagulation, treated with intracoronary stenting, without LAAO, collected from March 2014 through March 2021, a period that was partially coincidental with the inclusion period of patients with LAAO. Procedural data were obtained from the hospital registries and cath labs of participant centers.
The use of antithrombotic treatment and the indication for LAAO were left to the treating cardiologist's criterion. In all patients, closure device implantation was indicated for the primary prevention of thrombotic and hemorrhagic events. Exclusion criteria were a) formal contraindication to anticoagulant therapy; b) patient with previous percutaneous atrial appendage closure outside the PCI time frame specified in the study; c) LAAO indicated due to significant bleeding or thromboembolic event after initiation of post-PCI antithrombotic therapy; d) refusal to be included in the study or sign the written informed consent; e) impossibility to obtain the clinical follow-up. It is important to clarify that, being a real-life study as it was, patients with previous bleeding were included, but that, at the time, their cardiologists did not consider requesting a LAAO and thus, when the PCI was performed, they had the option of being assessed for LAAO. However, if a patient underwent LAAO before or after PCI due to bleeding, they were not considered study eligible.
Patients in the medical treatment group were all included consecutively at the coordination center to ensure data quality, as they were the largest group and could pose a greater challenge regarding follow-up. The presence of digitized medical records at regional level in the coordination center, and the thoroughness of follow-up ensured high-quality data collection for these patients.
Percutaneous coronary intervention and left atrial appendage closure
The indication for LAAO was established by the treating clinician after coronary anatomy was determined. LAAO was performed during the peri-PCI period (before, at the time of or within a 6-month time frame after PCI). The implantation technique, type of device, and post-implantation antithrombotic treatment were selected and left to the operator's criterion. As an indication IIb, the inclusion of these patients was limited and generally followed a strategy of avoiding the withdrawal of antiplatelet therapy and/or fear of bleeding with antithrombotic combination. They were consecutive but several months could pass between one and the other due to these circumstances.
Follow-up and outcome definitions
All patients were clinically followed after PCI, even in the arm in which the LAAO is subsequently performed. At follow-up, the appearance of the following events was prospectively collected: death, hemorrhage, stroke or transient ischemic attack, and acute myocardial infarction (AMI). Composite endpoints were defined as major adverse events (defined as the primary endpoint) including death, major bleeding or stroke/transient ischemic attack, and major adverse cardiovascular events including cardiac death, stroke/transient ischemic attack, and AMI. Hemorrhages were classified according to the Bleeding Academic Research Consortium (BARC) guidelines.10 Only BARC ≥ 2 hemorrhages classified as relevant, and BARC ≥ 3 hemorrhages as major (fatal bleeding, and/or symptomatic bleeding in a critical area or organ such as intracranial, intraspinal, intraocular, retroperitoneal, intra-articular, pericardial or intramuscular with compartment syndrome, and/or bleeding causing hemoglobin levels drop ≥ 2 g/L [1.24 mmol/L] or requiring transfusion of ≥ 2 units of whole blood or red cells) were recorded.
There was no loss in the LAAO group while only 5 patients from the medical treatment group (3.4%) were lost (without known event) before the study completion date.
Statistical methods
Continuous variables were expressed as mean ± SD or median (25th-75th percentiles) depending on data distribution. The categorical ones were compared using chi-square or Fisher's exact test while numerical variables were analyzed using the Student t test or the Mann-Whitney U test. The observed adjusted incidence rate regarding the density of events (number of events at follow-up divided by the sum of person-time of the at-risk population) are expressed as 100 patient-years. Event-free survival was analyzed using the Kaplan-Meier and Cox methods. All data were analyzed using the SPSS V.22.0 statistical software package.
Ethical aspects
The DESAFIO (DES implantation in patients with atrial fibrillation followed by LAA occlusion device) study protocol was approved by the independent ethics committees of the participant hospitals, and all patients gave their written informed consent to participate in this study. All procedures comply with the Declaration of Helsinki. The authors' center approved the data analysis. Registration was not deemed necessary since this is an observational study.
RESULTS
Overall, 146 patients received conventional antithrombotic treatment with OAC (75 with the vitamin K antagonist [VKA] acenocoumarol, and 71 with DOAC) while 61 patients underwent LAAO. The median follow-up after PCI was 35 months (figure 2).
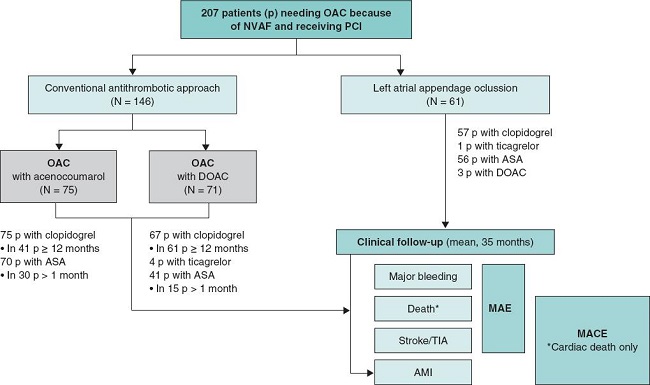
Figure 2. Study flowchart. AMI, acute myocardial infarction; ASA, acetylsalicylic acid; DOAC, direct oral anticoagulants; MACE, major adverse cardiovascular events; MAE, major adverse events; NVAF, non-valvular atrial fibrillation; OAC, oral anticoagulants; p, patients; PCI, percutaneous coronary intervention; TIA, transient ischemic attack.
The characteristics of both groups are shown on table 1, and classified according to their possible impact on ischemic-thrombotic or hemorrhagic events.
Table 1. Baseline characteristics.
| MT (N = 146) | Acenocoumarol (N = 75) | DOAC (N = 71) | LAAO (N = 61) | Pa | Pb | |
|---|---|---|---|---|---|---|
|
| ||||||
| Age | 75.7 ± 8.8 | 75.1 ± 8.8 | 76.4 ± 8.8 | 75.8 ± 8.9 | .947 | .664 |
|
| ||||||
| Age ≥ 75 years | 88 (60.3) | 41 (54.7) | 47 (66.2) | 36 (59.0) | .866 | .359 |
|
| ||||||
| Age, 65-74 years | 36 (24.7) | 23 (30.7) | 13 (18.3) | 17 (27.9) | .629 | .206 |
|
| ||||||
| Female sex | 41 (28.1) | 22 (29.3) | 19 (26.8) | 14 (23.0) | .446 | .703 |
|
| ||||||
| Thrombotic characteristics | ||||||
|
| ||||||
| Paroxysmal AF | 79 (54.1) | 38 (50.7) | 41 (57.7) | 27 (44.3) | .196 | .301 |
|
| ||||||
| Permanent AF | 66 (45.5) | 37 (49.3) | 30 (42.9) | 34 (55.7) | .180 | .249 |
|
| ||||||
| Chronic heart failure | 28 (19.2) | 15 (20.0) | 13 (18.3) | 18 (29.5) | .103 | .257 |
|
| ||||||
| High blood pressure | 121 (82.9) | 65 (86.7) | 56 (78.9) | 55 (90.2) | .180 | .171 |
|
| ||||||
| DM | 71 (48.6) | 33 (44.0) | 38 (53.5) | 24 (39.3) | .222 | .243 |
|
| ||||||
| History of stroke/TIA/TE | 27 (18.5) | 11 (14.7) | 16 (22.5) | 15 (24.6) | .320 | .303 |
|
| ||||||
| History of stroke/TIA | 24 (16.4) | 9 (12.0) | 15 (21.1) | 15 (24.6) | .172 | .145 |
|
| ||||||
| Previous CAD | 63 (43.2) | 36 (48.0) | 27 (38.0) | 46 (75.4) | < .001 | < .001 |
|
| ||||||
| Previous AMI | 25 (17.1) | 12 (16.0) | 13 (18.3) | 24 (39.3) | .001 | .003 |
|
| ||||||
| Previous PCI | 45 (30.8) | 27 (36.0) | 18 (25.4) | 42 (68.9) | < .001 | < .001 |
|
| ||||||
| Previous APE | 14 (9.6) | 5 (6.7) | 9 (12.7) | 14 (23.0) | .010 | .021 |
|
| ||||||
| Previous APE/AMI/revasc | 59 (40.4) | 32 (42.7) | 27 (38.0) | 48 (78.7) | < .001 | < .001 |
|
| ||||||
| CHADS2 | 2.48 ± 1.31 | 2.35 ± 1.24 | 2.62 ± 1.39 | 2.67 ± 1.34 | .340 | .292 |
|
| ||||||
| CHADS-VASc | 4.07 ± 1.70 | 3.92±1.68 | 4.11 ± 1.75 | 4.56 ± 1.53 | .033 | .082 |
|
| ||||||
| Bleeding characteristics | ||||||
|
| ||||||
| BP > 160 mmHg | 14 (9.6) | 8 (10.7) | 6 (8.5) | 10 (16.4) | .163 | .347 |
|
| ||||||
| Liver or kidney failure | 38 (26.0) | 23 (30.7) | 15 (21.1) | 22 (36.1) | .147 | .156 |
|
| ||||||
| Dialysis | 4 (2.7%) | 4 (5.3) | 0 | 7 (11.5) | .017 | .014 |
|
| ||||||
| Previous stroke/TIA | 24 (16.4) | 9 (12.0) | 15 (21.1) | 15 (24.6) | .172 | .145 |
|
| ||||||
| Previous bleeding | 13 (8.9) | 9 (12.0) | 4 (5.6) | 30 (49.2) | < .001 | < .001 |
|
| ||||||
| High bleeding risk | 29 (19.9) | 19 (25.3) | 10 (14.1) | 37 (60.7) | < .001 | < .001 |
|
| ||||||
| Labile INR | 10 (6.8) | 8 (10.7) | 2 (2.8) | 6 (9.8) | .463 | .158 |
|
| ||||||
| Age > 65 | 124 (84.9) | 64 (85.3) | 60 (84.5) | 53 (86.9) | .716 | .927 |
|
| ||||||
| Anti-inflammatory drugs | 9 (6.2) | 5 (6.7) | 4 (5.6) | 11 (18.0) | .008 | .030 |
|
| ||||||
| Alcohol/drug abuse | 5 (3.4) | 2 (2.7) | 3 (4.2) | 2 (3.3) | .999 | .872 |
|
| ||||||
| HAS-BLED | 1.63 ± 1.09 | 1.72 ± 1.24 | 1.54 ± 0.91 | 2.49 ± 1.18 | < .001 | < .001 |
Data from the groups in brackets are expressed as percentages. AF, atrial fibrillation; AMI, acute myocardial infarction; APE, acute pulmonary edema; BP, blood pressure; CAD, coronary artery disease; DM, diabetes mellitus; DOAC, direct oral anticoagulants; INR, international normalized ratio; LAAO, left atrial appendage. occlusion; MT, medical treatment; PCI, percutaneous coronary intervention; revasc, revascularization; TE, thromboembolism; TIA, transient ischemic attack.
aLAAO vs MT.
bLAAO vs acenocoumarol vs DOAC.
There were no significant differences in variables such as age (mean, 75.5 years), high blood pressure, diabetes, sex, permanent or paroxysmal AF or past medical history of stroke or thromboembolism between the 2 groups. There were, however, more unfavorable characteristics in the LAAO group with significant differences being reported in the past medical history of coronary disease (43.2% vs 75.4%; P < .001), CHA2DS2-VASc (4.07 ± 1.70 vs 4.56 ± 1.53; P = .033), relevant bleeding (BARC ≥ 2) (8.9% vs 49.2%; P < .001), high bleeding risk (defined as previous bleeding or HAS-BLED ≥ 3) (19.9% vs 62.3%; P < .001), and HAS-BLED score (1.63 ± 1.09 vs 2.49 ± 1.18; P < .001) between the OAC and the LAAO group, respectively. As shown on table 1, 36% of the patients from the LAAO group had GI bleeding vs 6.8% of the patients from the medical treatment group.
Table 2 shows the PCI-related characteristics of both groups, and table 3 the different bleeding types and their classification. A total of 41% of the patients from the COI group showed GI bleeding compared to 6.8% of the patients from the group on medical therapy as shown on table 3.
Table 2. Percutaneous coronary intervention: indications and type.
| MT (N = 146) | Acenocoumarol (N = 75) | DOAC (N = 71) | LAAO (N = 61) | Pa | Pb | |
|---|---|---|---|---|---|---|
|
| ||||||
| PCI indication | ||||||
|
| ||||||
| Stable angina | 15 (10.3) | 6 (8.0) | 9 (12.7) | 12 (19.7) | .067 | .132 |
|
| ||||||
| NSTEACS | 94 (64.4) | 55 (73.3) | 39 (54.9) | 43 (70.5) | .397 | .044 |
|
| ||||||
| STEACS | 37 (25.3) | 14 (18.7) | 23 (32.4) | 6 (9.8) | .012 | .005 |
|
| ||||||
| Number of diseased vessels | 1.76 ± 0.77 | 1.71 ± 0.71 | 1.82 ± 0.83 | 2.02 ± 1.06 | .091 | .116 |
|
| ||||||
| Number of vessels treated | 1.32 ± 0.52 | 1.36 ± 0.56 | 1.27 ± 0.48 | 1.30 ± 0.53 | .782 | .575 |
|
| ||||||
| Number of lesions treated | 1.50 ± 0.78 | 1.45 ± 0.72 | 1.55 ± 0.83 | 1.48 ± 0.77 | .835 | .736 |
|
| ||||||
| Number of stents | 1.71 ± 0.96 | 1.64 ± 0.78 | 1.75 ± 1.10 | 1.69 ± 1.04 | .984 | .827 |
|
| ||||||
| PCI on LMCA | 11 (7.5) | 7 (9.3) | 4 (5.6) | 6 (9.8) | .582 | .617 |
|
| ||||||
| PCI on proximal LAD | 37 (25.3) | 15 (20.0) | 23 (31.0) | 19 (31.1) | .391 | .227 |
|
| ||||||
| PCI on bifurcation | 3 (2.1) | 2 (2.7) | 1 (1.4) | 2 (3.3) | .601 | .772 |
|
| ||||||
| PCI due to restenosis/stent thrombosis | 3 (2.1) | 1 (1.3) | 2 (2.8) | 1 (1.6) | 1 | .793 |
|
| ||||||
| PCI due to overlapping stents | 20 (13.7) | 2 (2.7) | 18 (25.4) | 8 (13.1) | .911 | < .001 |
|
| ||||||
| PCI due to recurrent AMI | 2 (1.4) | 1 (1.3) | 1 (1.4) | 6 (9.8) | .009 | .016 |
Data from the groups in brackets are expressed as percentages. AMI, acute myocardial infarction; DES, drug-eluting stent; LAD, left anterior descending coronary artery; DOAC, direct oral anticoagulants; LAAO, left atrial appendage occlusion; LMCA, left main coronary artery; MT, medical treatment; NSTEACS, non-ST-segment elevation acute coronary syndrome; STEACS, ST-segment elevation acute coronary syndrome; PCI, percutaneous coronary intervention.
aLAAO vs MT.
bLAAO vs Acenocoumarol vs DOAC.
Table 3. Past medical history of bleeding prior to percutaneous coronary intervention in each group.
| MT (N = 146) | Acenocoumarol (N = 75) | DOAC (N = 71) | LAAO (N = 61) | Pa | Pb | |
|---|---|---|---|---|---|---|
|
| ||||||
| Characteristics | ||||||
|
| ||||||
| Need for transfusion | 5 (3.4) | 4 (5.3) | 1 (1.4) | 13 (21.3) | < .001 | < .001 |
|
| ||||||
| Need for admission | 6 (4.1) | 5 (6.7) | 1 (1.4) | 23 (37.7) | < .001 | < .001 |
|
| ||||||
| Hb drop by 3-5 g/dL | 4 (2.7) | 3 (4.0) | 1 (1.4) | 11 (18.0) | < .001 | < .001 |
|
| ||||||
| Hb drop > 5 g/dL | 0 | 0 | 0 | 5 (8.2) | .002 | .002 |
|
| ||||||
| BARC score | ||||||
|
| ||||||
| Type 2 | 9 (6.2) | 4 (5.3) | 3 (4.2) | 8 (13.1) | .097 | < .001 |
|
| ||||||
| Type 3a | 5 (3.4) | 5 (6.7) | 1 (1.4) | 10 (16.4) | .002 | < .001 |
|
| ||||||
| Type 3b | 0 | 0 | 0 | 6 (9.8) | .002 | < .001 |
|
| ||||||
| Type 3c | 0 | 0 | 0 | 6 (9.8) | .001 | .004 |
|
| ||||||
| Type of bleeding | ||||||
|
| ||||||
| Intracranial | 0 | 0 | 0 | 6 (9.8) | .001 | .001 |
|
| ||||||
| GI | 10 (6.8) | 8 (10.7) | 2 (2.8) | 25 (41.0) | < .001 | < .001 |
|
| ||||||
| Other | 3 (2.1) | 1 (1.3) | 2 (2.8) | 2 (3.3) | .267 | .463 |
Data from the groups in brackets are expressed as percentages. BARC, Bleeding Academic Research Consortium guidelines; DES, drug-eluting stent; DOAC, direct oral anticoagulants; GI, gastrointestinal; Hb, hemoglobin; LAAO, left atrial appendage occlusion; MT, medical treatment; PCI, percutaneous coronary intervention.
aLAAO vs MT.
bLAAO vs acenocoumarol vs DOAC.
In the intervention group, this was the timeline association between LAAO and PCI: in 4 (6.6%) patients, LAAO was performed a median of 35 days before PCI; in 4 (6.6%), during the same procedure, while in 53 patients (86.9%), it was performed a median of 75 days after the PCI. The Amplatzer Amulet (Abbott, United States), WATCHMAN (Boston Scientific, United States), and LAmbre (Lifetech Scientific, China) devices were used in 50 (82%), 9 (14.8%), and 2 (3.2%) patients, respectively.
Regarding the antithrombotic regimen used between the PCI and LAAO, 30 patients received triple antiplatelet therapy while 23 received DAPT. After LAAO, most remained on DAPT (table 4).
Table 4. Antiplatelet therapy after percutaneous coronary intervention or left atrial appendage closure.
| Acenocoumarol (N = 75) | DOAC (N = 71) | LAAO (N = 61) | P | |
|---|---|---|---|---|
|
| ||||
| ASA | 70 (93.3) | 41 (57.7) | 56 (91.8) | < .001 |
|
| ||||
| ASA > 1 month | 39 (57.1) | 15 (29.7) | 31 (50.8) | < .001 |
|
| ||||
| Clopidogrel | 75 (100) | 67 (94.4) | 57 (93.4) | .091 |
|
| ||||
| Clopidogrel ≥ 6 months | 41 (57.7) | 61 (92.4) | 28 (57.1) | < .001 |
|
| ||||
| Ticagrelor | 0 | 4 (5.6) | 1 (1.6) | .077 |
|
| ||||
| DOAC | 0 | 71 (100) | 3 (4.9) | - |
|
| ||||
| 1 month-triple antiplatelet therapy | 39 (57.1) | 15 (29.7) | 0 | < .001 |
|
| ||||
| 6 month-triple antiplatelet therapy | 3 (4) | 5 (7) | 0 | < .001 |
|
| ||||
| DT > 6 months | 54 (72) | 65 (91.5) | 1 (1.6) | < 0.001 |
Data from the groups in brackets are expressed as percentages. ASA, acetylsalicylic acid; DOAC, direct oral anticoagulants; DT, double therapy (anticoagulation + antiplatelet); LAAO, left atrial appendage occlusion.
Clinical outcomes
The rates of major adverse events and major adverse cardiovascular events were significantly higher in the OAC group: 19.75% vs 9.06% (HR, 2.18; P = .008) and 6.37% vs 1.91% (HR, 3.34; P = .037) (table 5, figure 3A), respectively. The median follow-up was 29.6 and 23.3 months for the medical and LAAO groups, respectively (table 6).
Table 5. Major adverse events and major adverse cardiovascular events at follow-up.
| MT (N = 146; 449 p-y) | Acenocoumarol (N = 75; 277 p-y) | DOAC (N = 71; 175 p-y) | LAAO (N = 61; 158 p-y) | HR (95%CI) | Pa | Pb | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Overall death | 49 (10.84) | 35 (12.68) | 14 (7.99) | 11 (6.95) | 1.56 (0.81-3.01) | .184 | .081 |
|
| |||||||
| Cardiac death | 4 (0.89) | 2 (0.72) | 2 (1.14) | 1 (0.63) | 1.41 (0.17-14.05) | .691 | .850 |
|
| |||||||
| Stroke/TIA | 19 (4.59) | 11 (4.31) | 8 (5.05) | 2 (1.27) | 3.59 (0.84-15.54) | .084 | .150 |
|
| |||||||
| AMI | 4 (0.91) | 4 (1.52) | 0 | 1 (0.63) | 1.44 (0.19-15.40) | .628 | .167 |
|
| |||||||
| PCI | 7 (1.68) | 5 (2.08) | 2 (1.14) | 1 (0.63) | 2.67 (0.29-20.09) | .415 | .314 |
|
| |||||||
| Bleeding BARC ≥ 2 | 42 (11.56) | 28 (13.14) | 14 (9.33) | 7 (4.57) | 2.53 (1.22-6.05) | .014 | .002 |
|
| |||||||
| Bleeding BARC ≥ 3 | 31 (7.99) | 22 (9.48) | 9 (5.77) | 6 (3.88) | 2.06 (0.93-5.36) | .072 | .011 |
|
| |||||||
| Overall death/stroke - TIA/BARC ≥ 2 bleeding | 76 (23.52) | 47 (24.54) | 29 (21.79) | 15 (9.80) | 2.40 (1.38-4.17) | .002 | .001 |
|
| |||||||
| Overall death/stroke - TIA/bleeding BARC ≥ 3 (MAE) | 68 (19.75) | 43 (20.43) | 25 (18.02) | 14 (9.06) | 2.18 (1.23-3.88) | .008 | .004 |
|
| |||||||
| Cardiac death/stroke/TIA/AMI (MACE) | 25 (6.37) | 15 (6.16) | 10 (6.32) | 3 (1.91) | 3.34 (1.01-11.06) | .037 | .069 |
Data from the groups in brackets are expressed as percentages. Absolute values and percentages are expressed per 100 patient-years. AMI, acute myocardial infarction; DOAC, direct oral anticoagulants; BARC, Bleeding Academic Research Consortium guidelines; LAAO, left atrial appendage occlusion; MACE, major adverse cardiovascular events; MAE, major adverse events; p, patients; PCI, percutaneous coronary intervention TIA, transient ischemic attack; y, years.
aMT vs LAAO.
bLAAO vs acenocoumarol vs DOAC.

Figure 3. Kaplan-Meier curves for major adverse events and major cardiovascular events-free survival (A) and bleeding events-free survival (B) at follow-up. AMI, acute myocardial infarction; BARC, Bleeding Academic Research Consortium; CI, confidence interval; HR, hazard ratio; LAAO, left atrial appendage occlusion; MACE, major adverse cardiovascular events; MAE, major adverse events; TIA, transient ischemic attack.
Table 6. Rates of events at 12 and 36 months.
| TM (146 p) | LAAO (61 p) | |
|---|---|---|
|
| ||
| MAE at 12 m | 37 (30.5) | 8 (14.13) |
|
| ||
| MAE at 36 m | 54 (20.5) | 13 (10.8) |
|
| ||
| Overall MAE | 68 (19.75) | 14 (9.06) |
|
| ||
| MACE at 12 m | 11 (8.28) | 2 (3.41) |
|
| ||
| MACE at 36 m | 18 (5.92) | 3 (2.43) |
|
| ||
| Overall MACE | 25 (6.37) | 3 (1.91) |
Rates are expressed as absolute number of events (100 patient-years). Data from the groups in brackets are expressed as percentages. m, months; MACE, major adverse cardiovascular events; MAE, major adverse events; p, patients.
The rates of death, stroke/acute cerebrovascular event, and relevant bleeding, expressed as 100 patient-years, were higher in the group of patients treated with OAC compared to the LAAO group. Since the rate of stroke was higher than expected in the OAC group, the possible reasons for this observation were further investigated. Out of the 19 patients reported with stroke, at least, 13 had some predisposing condition that could have increased risk: a) treatment withdrawal due to surgery: 3 cases; b) treatment withdrawal due to bleeding: 2 cases; and c) under-dosing: 9 cases (4 of which were in the VKA group with an international normalized ratio < 2).
Figure 3B shows significant bleeding differences between the LAAO and the OAC group. Table 5 shows bleeding events by group; a favorable trend was found in the LAAO group even compared to DOAC regarding relevant bleeding.
In the multivariate analysis (Cox regression), only the HAS-BLED score (HR, 1.30; 95%CI, 1.04-1.62; P = .019) and medical treatment allocation (HR, 3.42; 95%CI, 1.57-7.42; P = .002) were independent predictors of major adverse events. On the other hand, the CHA2DS2-VASc score (HR, 1.24; 95%CI, 1.01-1.53; P = .043), and medical treatment allocation (HR, 3.71; 95%CI, 1.11-12.37; P =.033) were independent predictors of major adverse cardiovascular events.
In the LAAO population the following procedural complications were reported: 1 patient with an arteriovenous fistula who did not require vascular surgery, 2 patients with pericardial effusion, 1 patient who required pericardiocentesis, and 1 patient with bronchospasm after extubation that resolved uneventufully with medical treatment.
DISCUSSION
Out study main finding was that, in patients with NVAF treated with coronary stents, LAAO plus AP showed better long-term outcomes compared to OAC (including DOAC) plus AP. These findings are significant considering the adverse characteristics of the LAAO group. The benefit of LAAO was maintained against both the acenocoumarol and DOAC subgroups. Regarding safety, there were significantly fewer hemorrhages (BARC 2 and 3) with LAAO compared to the acenocoumarol group. No significant differences regarding hemorrhages were reported between the LAAO and the DOAC group although there were fewer events in the LAAO arm, especially BARC ≥ 2.
In recent years, 4 studies on DOAC and several meta-analyses showed that DAPT (DOAC plus P2Y12, usually clopidogrel) is associated with fewer hemorrhages compared to triple antiplatelet therapy (warfarin + clopidogrel + aspirin). Also that this treatment is rarely associated with worse outcomes in ischemic-thrombotic events.11-16 These results are undoubtedly important and have shaped the new recommendations published by scientific societies on the management of these patients.17
However, bleeding rates remain very high in this population. In addition, in former studies, the combination of VKAs plus aspirin has already been a less effective strategy compared to DAPT.1
The major PIONEER11 and REDUAL14 studies reported a mean annual rate of bleeding after a 12-month follow-up with DAPT consisting of DOAC plus clopidogrel of 16.9% and 20.2%, respectively. Of note, data from the AUGUSTUS trial on apixaban are limited to 6 months only, which would explain, at least partially, the lower rate of bleeding reported.11,12,14,15
Earlier studies comparing DAPT with aspirin and clopidogrel in patients without AF showed significantly lower rates of bleeding compared to the combination of VKAs plus aspirin.18
The clinical follow-up of most DOAC studies has been short (from the 6 months of the AUGUSTUS15 trial to the 14 months of the REDUAL14). Our study reported on a 35-month follow-up. Although bleeding events are known to be more common within the first year for both groups, we saw that beyond the first year, curves diverged favoring the LAAO group (figure 3B). This had already been shown in large LAAO registries.19 As these treatments are lifelong, the risk of recurrent bleeding and the possibility of future surgical procedures in patients > 70-75 years who need to stop using OAC underline the need for assessing other possible therapeutic alternatives. The rate of thromboembolic events, especially stroke, is known to be significant within the first few days after OAC discontinuation.20
Similarly, recurrent ACS in patients who have already had coronary events is not rare. In the Melbourne registry of 9615 patients, 12% required hospitalization 1 year after ACS.21 Some of these patients require a new PCI, which again brings back the dilemma of using a combined treatment or not.
Importantly, the exclusion criteria specified in these studies limit the applicability of results to the general population of hospitalized patients. It is estimated that the results of these trials could be generalized to less than two-thirds of the patients in the routine clinical practice.22
In most studies of DOAC in patients with AF undergoing PCI, the history of previous bleeding is not recorded except for, indirectly, in patients with a past medical history of previous GI bleeding and, in any case, these patients are largely misrepresented. Thus, 1.3% had a past medical history of GI bleeding in the DOAC plus clopidogrel subgroup of the PIONEER study compared to 5% in the warfarin plus clopidogrel group of the WOEST study.14,23 In our study, 10.7%, 2.8%, and 41% of the patients from the OAC, DOAC, and LAAO groups, respectively, had a past medical history of GI bleeding. Unsurprisingly, since the study was not randomized, cardiologists ordered the LAAO strategy more frequently in patients with a past medical history of bleeding.
Although these 4 studies do report HAS-BLED scores, the predictive value of this parameter, while of use, is much lower compared to the past medical history of bleeding, especially in patients with a history of bleeding and age > 75 years as seen in large LAAO studies.24,25
Finally, ischemic events showed significance favorable to the DAPT group vs dual antithrombotic therapy (1.6% vs 6.2%; P = .01; and 0.5% vs 2.7%: P = .01), respectively.1
Limitations
The number of patients was small, and our study was not randomized. Therefore, our results should only be considered hypothesis generating in this pilot study. Despite being an observational study with no control of confounding bias in its design, most of the baseline variables were equally distributed among the groups being the hemorrhagic and thrombotic risks reported at baseline even more unfavorable in the LAAO group. However, a selection bias cannot be ruled out in patients from the LAAO group.
The rate of stroke in the medical treatment group was higher than expected from pivotal studies. This was probably the result of the associated comorbidities that increase the likelihood of readmissions due to invasive procedures that, in turn, require anticoagulant therapy modification as a bridging therapy to these procedures. This increases the long-term rate of stroke in this population compared to those who have undergone LAAO. However, it reflects real-world. Further studies are required to elucidate what the best therapeutic strategy is for these challenging patients.
CONCLUSIONS
In patients with NVAF treated with coronary stents, an LAAO strategy with AP provides superior long-term results regarding major adverse events and major adverse cardiovascular events compared to treatment with OAC (DOAC included) plus AP despite the more unfavorable characteristics reported in the LAAO group.
The benefit favorable to the LAAO group persisted over both VKA and DOAC groups. There were significantly fewer hemorrhagic events (BARC 2 and 3) following LAAO compared to the VKA group, but not between LAAO and DOAC (although there were fewer events in the LAAO arm, especially BARC ≥ 2).
WHAT IS KNOWN ABOUT THE TOPIC?
- There is growing evidence that LAAO can be a potential therapeutic alternative to the use of OAC in patients with NVAF and a history of significant bleeding or high bleeding risk.
- LAAO has similar efficacy to OAC preventing the occurrence of thromboembolisms and associates minor bleeding risk especially 1 year after the procedure.
WHAT DOES THIS STUDY ADD?
- The study results suggest that LAAO is a favorable alternative to OAC in patients with NVAF with ischemic heart disease who require coronary stenting and AP-based therapies.
- The observations summarized herein demonstrate, in a real-world setting, that the combination of LAAO plus AP results in a lower rate of major adverse events and major adverse cardiovascular events compared to treatment with OAC plus AP.
ACKNOWLEDGEMENTS
Medical writing support was provided by Javier Arranz-Nicolás, PhD, from Medical Statistics Consulting (MSC), Valencia, Spain under the authors' guidance.
Abbreviations
AP: |
antiplatelet drugs; |
LAAO: |
left atrial appendage occlusion; |
NVAF: |
non-valvular atrial fibrillation; |
OAC: |
oral anticoagulants; |
PCI: |
percutaneous coronary intervention; |
TIA: |
transient ischemic attack. |
REFERENCES
1. Leon MB, Baim DS, Popma JJ, et al. A Clinical Trial Comparing Three Antithrombotic-Drug Regimens after Coronary-Artery Stenting. N Engl J Med. 1998;339:1665-1671. [ Links ]
2. Benjamin EJ, Virani SS, Callaway CW, et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation. 2018;137:e67-e492. [ Links ]
3. Kralev S, Schneider K, Lang S, Süselbeck T, Borggrefe M. Incidence and severity of coronary artery disease in patients with atrial fibrillation undergoing first-time coronary angiography. PLoS One. 2011;6:e24964. [ Links ]
4. González-Pacheco H, Márquez MF, Arias-Mendoza A, et al. Clinical features and in-hospital mortality associated with different types of atrial fibrillation in patients with acute coronary syndrome with and without ST elevation. J Cardiol. 2015;66:148-154. [ Links ]
5. Rohla M, Vennekate CK, Tentzeris I, et al. Long-term mortality of patients with atrial fibrillation undergoing percutaneous coronary intervention with stent implantation for acute and stable coronary artery disease. Int J Cardiol. 2015;184:108-114. [ Links ]
6. Généreux P, Giustino G, Witzenbichler B, et al. Incidence, Predictors, and Impact of Post-Discharge Bleeding After Percutaneous Coronary Intervention. J Am Coll Cardiol. 2015;66:1036-1045. [ Links ]
7. Busu T, Khan SU, Alhajji M, Alqahtani F, Holmes DR, Alkhouli M. Observed versus Expected Ischemic and Bleeding Events Following Left Atrial Appendage Occlusion. Am J Cardiol. 2020;125:1644-1650. [ Links ]
8. Osmancik P, Herman D, Neuzil P, et al. 4-Year Outcomes After Left Atrial Appendage Closure Versus Nonwarfarin Oral Anticoagulation for Atrial Fibrillation. J Am Coll Cardiol. 2022;79:1-14. [ Links ]
9. Reddy VY, Doshi SK, Kar S, et al. 5-Year Outcomes After Left Atrial Appendage Closure. J Am Coll Cardiol.. 2017;70:2964-2975. [ Links ]
10. Mehran R, Rao SV, Bhatt DL, et al. Standardized Bleeding Definitions for Cardiovascular Clinical Trials. Circulation. 2011;123:2736-2747. [ Links ]
11. Cannon CP, Bhatt DL, Oldgren J, et al. Dual Antithrombotic Therapy with Dabigatran after PCI in Atrial Fibrillation. N Eng J Med. 2017;377: 1513-1524. [ Links ]
12. Capodanno D, Huber K, Mehran R, et al. Management of Antithrombotic Therapy in Atrial Fibrillation Patients Undergoing PCI. J Am Coll Cardiol. 2019;74:83-99. [ Links ]
13. Gargiulo G, Goette A, Tijssen J, et al. Safety and efficacy outcomes of double vs triple antithrombotic therapy in patients with atrial fibrillation following percutaneous coronary intervention: a systematic review and meta-analysis of non-vitamin K antagonist oral anticoagulant-based randomized clinical trials. Eur Heart J. 2019;40:3757-3767. [ Links ]
14. Gibson CM, Mehran R, Bode C, et al. Prevention of Bleeding in Patients with Atrial Fibrillation Undergoing PCI. N Eng J Med. 2016;375:2423-2434. [ Links ]
15. Lopes RD, Leonardi S, Wojdyla DM, et al. Stent Thrombosis in Patients With Atrial Fibrillation Undergoing Coronary Stenting in the AUGUSTUS Trial. Circulation. 2020;141:781-783. [ Links ]
16. Vranckx P, Valgimigli M, Eckardt L, et al. Edoxaban-based versus vitamin K antagonist-based antithrombotic regimen after successful coronary stenting in patients with atrial fibrillation (ENTRUST-AF PCI): a randomised, open-label, phase 3b trial. Lancet. 2019;394:1335-1343. [ Links ]
17. Cheung CC, Nattel S, Macle L, Andrade JG. Management of Atrial Fibrillation in 2021: An Updated Comparison of the Current CCS/CHRS, ESC, and AHA/ACC/HRS Guidelines. Can J Cardiol. 2021;37:1607-1618. [ Links ]
18. Bertrand ME, Legrand V, Boland J, et al. Randomized Multicenter Comparison of Conventional Anticoagulation Versus Antiplatelet Therapy in Unplanned and Elective Coronary Stenting. Circulation. 1998;98:1597-1603. [ Links ]
19. López-Mínguez JR, Nogales-Asensio JM, Infante De Oliveira E, et al. Long-term Event Reduction After Left Atrial Appendage Closure. Results of the Iberian Registry II. Rev Esp Cardiol. 2019;72:449-455. [ Links ]
20. Yao X, Abraham NS, Alexander GC, et al. Effect of Adherence to Oral Anticoagulants on Risk of Stroke and Major Bleeding Among Patients With Atrial Fibrillation. J Am Heart Assoc. 2016;5:e003074. [ Links ]
21. Yudi MB, Clark DJ, Farouque O, et al. Trends and predictors of recurrent acute coronary syndrome hospitalizations and unplanned revascularization after index acute myocardial infarction treated with percutaneous coronary intervention. Am Heart J. 2019;212:134-143. [ Links ]
22. Lee S, Monz BU, Clemens A, Brueckmann M, Lip GYH. Representativeness of the dabigatran, apixaban and rivaroxaban clinical trial populations to real-world atrial fibrillation patients in the United Kingdom: a cross-sectional analysis using the General Practice Research Database. BMJ Open. 2012;2:e001768. [ Links ]
23. Dewilde WJM, Oirbans T, Verheugt FWA, et al. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet. 2013;381:1107-1115. [ Links ]
24. López-Mínguez JR, Nogales-Asensio JM, Infante De Oliveira E, et al. Major Bleeding Predictors in Patients with Left Atrial Appendage Closure: The Iberian Registry II. J Clin Med. 2020;9:2295. [ Links ]
25. Tarantini G, D'Amico G, Schmidt B, et al. The Impact of CHA2DS2-VASc and HAS-BLED Scores on Clinical Outcomes in the Amplatzer Amulet Study. JACC: Cardiovasc Interv. 2020;13:2099-2108. [ Links ]
Received: February 06, 2023; Accepted: April 19, 2023; pub: July 26, 2023











 text in
text in 


