Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista de Osteoporosis y Metabolismo Mineral
versión On-line ISSN 2173-2345versión impresa ISSN 1889-836X
Rev Osteoporos Metab Miner vol.7 no.4 Madrid nov./dic. 2015
https://dx.doi.org/10.4321/S1889-836X2015000400004
BMD evolution during treatment with aromatase inhibitors and its relation to the CYP11A1 gene: prospective study in the B-ABLE cohort
Evolución de la DMO durante el tratamiento con inhibidores de aromatasa y su relación con el gen CYP11A1: estudio prospectivo de la cohorte B-ABLE
Rodríguez-Sanz M.1, Prieto-Alhambra D.1,3,4,5, Servitja S.6, García-Giralt N.1, Garrigos L.6, Albanell J.6, Martínez-García M.6, González I.6, Martos T.6, Díez-Pérez A.1,2, Tusquets I.6, Nogués X.1,2
1 IMIM (Instituto Hospital del Mar de Investigaciones Médicas) - Red Temática de Investigación Cooperativa en Envejecimiento y Fragilidad (RETICEF) - Instituto de Salud Carlos III FEDER - Barcelona (España)
2 Departamento de Medicina Interna - Parque de Salud Mar - Universidad Autónoma de Barcelona - Barcelona (España)
3 IDIAP (Instituto de Investigación en Atención Primaria Jordi Gol) - Universidad Autónoma de Barcelona - Barcelona (España)
4 Departamento de Ortopedia - Reumatología y Ciencias Musculoesqueléticas Nuffield - Unidad de Investigación Biomédica y Musculoesquelético de NIHR - Universidad de Oxford - Oxford (Reino Unido)
5 MRC Unidad de Epidemiología del Ciclo Vital de la Universidad de Southampton - Southampton (Reino Unido)
6IMIM (Instituto Hospital del Mar de Investigaciones Médicas) - Departamento de Oncología Médica - Parque de Salud Mar - Barcelona (España)
Work scholarship from the FEIOMM to attend the 35th Congress of the ASBMR (Baltimore, 2013).
This work was supported by the Thematic Research Network Cooperative Aging and Fragility (RETICEF; RD12/0043/0022), and aid FIS PI10/01464 and PI13/00444 (Carlos III Health Institute, Ministry of Science and Innovation). The Generalitat of Catalonia (DIUE 2014 SGR 775) and ERDF funds have also contributed to its financing.
SUMMARY
Objectives: The aim of this study was to analyze bone mineral density (BMD) changes throughout aromatase inhibitor (AI) treatment in clinical cases and also consider its association with the CYP11A1 gene and the BMD variation after treatment.
Material and methods: The B-ABLE cohort is a prospective study of postmenopausal women with breast cancer, in AI treatment. BMD variation was analyzed during AI treatment, as well as the differences those patients who were treated and not treated previously with tamoxifen (TMX). Three polymorphisms (rs4077581, rs11632698 and rs900798) of the CYP11A1 gene were genotyped for their association with BMD variation.
Results: TMX-treated patients presented more rapid BMD loss than those who did not undergo prior TMX treatment (60% less in spine and 46% in femur at 2 years and 70% less in the spine and 63% in the femur at 3 years). However, no significant BMD loss was detected after treatment in either group. The 3 CYP11A1 gene polymorphisms were significantly associated with BMD variation in the femur at the end of the treatment.
Conclusions: BMD was reduced more rapidly in patients with prior TMX treatment than in those who only received AI, although no significant differences were detected after treatment. The 3 CYP11A1 gene polymorphisms were associated with BMD variation in response to AI treatment.
Key words: aromatase inhibitors, bone mineral density, CYP11A1, genetic polymorphisms, tamoxifen.
RESUMEN
Objetivos: El objetivo del estudio fue analizar los cambios en la densidad mineral ósea (DMO) a lo largo del tratamiento con inhibidores de aromatasa (IA) en la práctica clínica y evaluar la asociación entre el gen CYP11A1 y la variación de DMO al final del tratamiento.
Material y métodos: La cohorte B-ABLE es un estudio prospectivo de mujeres postmenopáusicas con cáncer de mama, en tratamiento con IA. Se analizó la variación de DMO durante todo el tratamiento con IA, así como las diferencias entre las pacientes tratadas y no-tratadas previamente con tamoxifeno (TMX). Tres polimorfismos (rs4077581, rs11632698 y rs900798) del gen CYP11A1, fueron genotipados para su asociación con la variación de DMO.
Resultados: Las pacientes tratadas con TMX mostraron pérdidas más aceleradas de DMO que las no tratadas previamente con TMX (60% menos en columna y 46% en fémur a los 2 años y 70% menos en columna y 63% en fémur a los 3 años). No obstante, al final del tratamiento no se detectaron diferencias significativas en la pérdida de DMO entre ambos grupos de pacientes. Los 3 polimorfismos del gen CYP11A1 resultaron significativamente asociados a la variación de DMO en fémur al final del tratamiento.
Conclusiones: La DMO disminuyó de forma más acelerada en las pacientes con tratamiento previo con TMX que en las que solo recibieron AI, a pesar de que no se detectaron diferencias significativas al final de tratamiento. Polimorfismos en el gen CYP11A1 están relacionados con la variación de la DMO en respuesta al tratamiento con IA.
Palabras clave: inhibidores de aromatasa, densidad mineral ósea, CYP11A1, polimorfismos genéticos, tamoxifeno.
Introduction
Aromatase inhibitors (AI) have become the accepted adjuvant therapy for postmenopausal patients with breast cancer with hormonal receptor expression [1]. AI brought about a marked reduction in estrogen levels through inhibition of the aromatase enzyme [2] whose activity is relegated to peripheral tissues during menopause [3]. The American Society for Clinical Oncology (ASCO) recommends using the AI for 5 years, or for 2 or 3 years, after previous therapy with tamoxifen (TMX) [4], where the latter option is prescribed for pre/peri-menopausal women [5].
However, reduced estrogen levels increase bone resorption and raise the risk of fracture that occurs after menopause [1,6-9]. Clinical guidelines for the management of bone loss associated with AI (AIBL: Aromatase Inhibitor associated Bone Loss) recommends a strict monitoring of bone mineral density (BMD) and other risk factors to assess the need for treatment with anti-resortive therapies [10].
Despite existing data, most of which based on randomized clinical trials (RCT), there is little information on the effect of AI therapy in routine clinical practice, where patient characteristics and adherence to therapy may differ from what is observed in restrictive RCT conditions.
The study data presented are taken from the B-ABLE cohort, a prospective clinical cohort in postmenopausal women with early stage breast cancer receiving adjuvant AI therapy. A recent study [11] in this cohort reported a large inter-individual variability in the change of BMD during AI treatment: at 2 years of therapy, more than 40% of patients experienced more than 3% BMD loss, while 20% of women did not present significant losses or even gained BMD. Moreover, in this same study, an association was found between CYP11A1 gene polymorphisms and bone loss after 2 years of AI treatment [11], thus demonstrating that the observed variability among patients presenting AIBL 2 years after treatment could be partially determined by genetics. The study aimed to describe BMD changes over the entire treatment, up to completion, and to assess the possible association between the CYP11A1 gene and AIBL after treatment.
Material and methods
Study Population
Details of the study design, methods of recruitment and population study [12] have been previously described and here are set out briefly.
B-ABLE is a prospective, observational clinical cohort study, initiated in January 2006 and currently with open inclusion. The women included presented postmenopausal breast cancer with hormone receptor expression, candidates for AI treatment and attending the Outpatient Breast Cancer Unit of the Hospital del Mar (Barcelona, Spain). Exclusion criteria are any history of bone disease, rheumatoid arthritis, endocrine and metabolic diseases or use of oral corticosteroids or any other drug with bone action, except Tamoxifen.
Procedures
Participants were treated with AI (Letrozole, Exemestane or Anastrozole) for 5 years, or alternatively after 2 or 3 years of Tamoxifen treatment (3 and 2 years of AI, respectively), according to ASCO recommendations [4] of starting within 6 weeks after surgery or 1 month after the last cycle of chemotherapy.
All participants received calcium and vitamin D (1,000 mg 800 IU daily), and those with vitamin D deficiency at baseline (<30 ng/ml) received an extra dose of 16,000 IU of Cholecalciferol oral every 2 weeks.
Measurements
Bone Mineral Density
At baseline and annually until the end of treatment, BMD was measured in lumbar spine (LS; L1-L4), femoral neck (FN) and total hip using the Densitometer X-ray Absorptiometry (DXA) SL® QDR 4500 (Hologic, Waltham, Massachusetts, USA), following our unit's standard protocol. In our department, the coefficient of variation for this technique ranges between LS 1% to 1.65% in FN. The images were scrutinized rigorously, especially in the interpretation of tracking scanners. Those who presented artifacts in the image, causing possible erroneous BMD increase (degenerative disc disease with bone spurs, arthritis with hyperostosis of the facet joints, vertebral fractures and/or aortic calcifications) were excluded from the analysis, in accordance with the description of Blake et al. [13]
Other determining
Information of a large number of clinical variables was collected at the time of recruitment, including age, age at menarche and menopause, nursing time, parity, previous chemotherapy and radiotherapy, adjuvant treatments, weight, height, serum levels of 25-hydroxyvitamin D (25 (OH) D), calcium intake and smoking.
Selection of candidate genes and polymorphisms
To study the association with AIBL at the end of treatment, we selected rs4077581 single-nucleotide polymorphisms (in the promoter region), rs11632698 (in intron 2) and rs900798 (in the 3'UTR) of the CYP11A1 gene, which have been previously associated with AIBL after 2 years of treatment [11].
DNA extraction and genotyping polymorphisms
DNA extraction was carried out on peripheral blood in LGC Genomics Units. Polymorphism genotyping was carried out using the Kaspar Genotyping System v4.0, at LGC Genomics. To verify quality of service, polymorphisms were also genotyped in a plate control consisting of a random sample containing 5% of total samples. The results showed 100% concordance.
Declaration of Ethics
Study protocols were approved by the appropriate ethics committee (Ethics Committee for Clinical Research of the Parc de Salut Mar [CEIC-Parc de Salut Mar]). Approved protocols for obtaining DNA from blood samples were explained, patients signed an informed consent before being included in the study.
Statistical analysis
Hardy-Weinberg equilibrium (HWE) was calculated by the online tool Tufts University Somerville/Medford, Massachusetts, USA [http://www.tufts.edu/~mcourt01/Documents/Court lab HW calculator.xls]. The outcome variable was BMD loss, calculated as the cumulative percentage change in BMD in LS and FN at each follow-up visit until the end of treatment (three or five years of treatment, as they had received tamoxifen previously). The patient group completing AI therapy after 2 years is not taken into account on an isolated basis when assessing BMD development, as the limited number of patients would not allow for statistical inference in this subset. BMD changes from baseline were assessed using Student's t-paired samples.
The association between polymorphisms and AIBL elected at the end of treatment. It was analyzed using multiple linear regression models contemplating heritage dominant, recessive and additive genetic. Analyses were adjusted for age, index body mass, chemotherapy and/or prior radiotherapy, tamoxifen therapy prior, initial BMD and years of treatment with AI. Furthermore it was also studied potential confusion about the levels of 25 (OH) D at the beginning and by the type of AI. For will minimize false findings because of multiple comparisons was used the FDR [14] correction, accepting those predictions with q <0.05 significant. Statistical analyzes were performed using R for Windows Version 2.13.2 (Packages: SNPassoc, foreign, multtest, boot and ggplot2).
Results
Baseline characteristics of patients and study AIBL
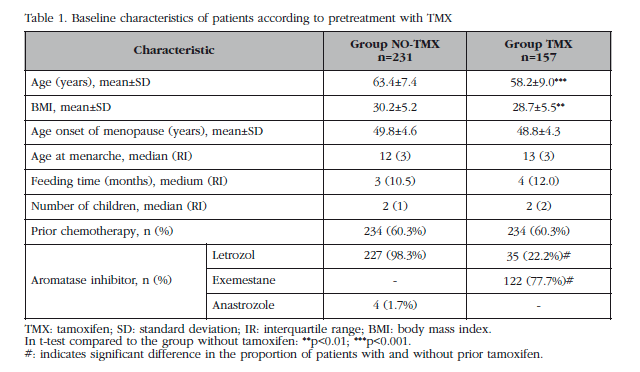
A total of 529 women were recruited from March 2006 to February 2013, of which 24.2% had a normal T-score, 57.3% were osteopenic and the remaining 18.5%, osteoporotic. A total of 388 (73.3%) patients did not receive bisphosphonate treatment, and thus were selected for analysis. A 40.5% of these patients (157) had received prior therapy with tamoxifen (TMX Group), while the remaining 60.5% were not treated with any prior hormonal therapy (NO-TMX Group). Clinical baseline features of the participants according to previous treatment with tamoxifen are shown in Table 1. Significant differences were detected between groups in age, BMI (body mass index) and type of AI.
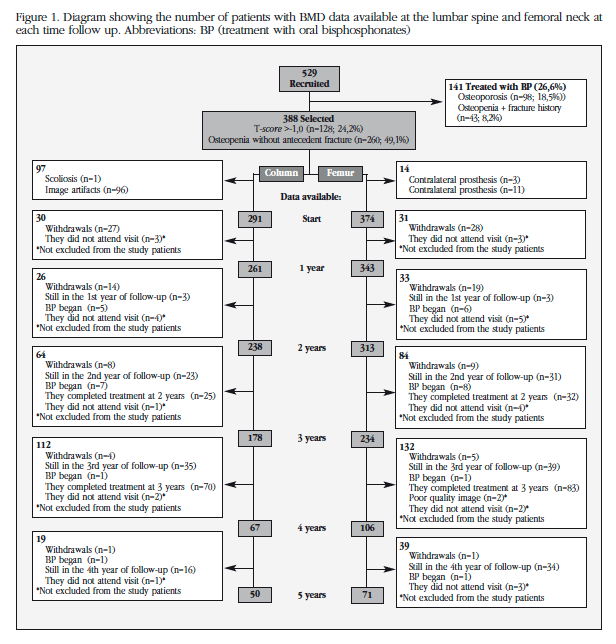
Figure 1 shows the number of patients with available data for LS and FN in each of the follow-up times. Patients with devices in the lumbar scanner and/or scoliosis (n=97) and those with artifacts in the hip scanner and/or bilateral prosthesis (n=14) were excluded for analysis for BMD of LS and FN, respectively. Of the 388 patients included in the analysis, 18 were reclassified as osteoporosis by decreasing BMD during treatment (7 in the first year, 8 during the second year, 1 during the third year and 2 in the fourth year) which were immediately implemented bisphosphonate therapy. From that point, their data were excluded from analysis.
Table 2 shows the absolute values of baseline BMD and end of treatment (3 or 5 years in the TMX and TMX NO-group, respectively). At baseline the TMX patients showed a higher BMD in CF patients that NO-TMX (+0,021 g/cm2 [95% CI 0.004 to 0.038]; p <0.05). No significant differences were detected in the initial LS BMD. No significant differences were detected in FN or LS BMD between 3 years or values between the values after treatment (3 years, 5 years vs the TMX group TMX NO-group).
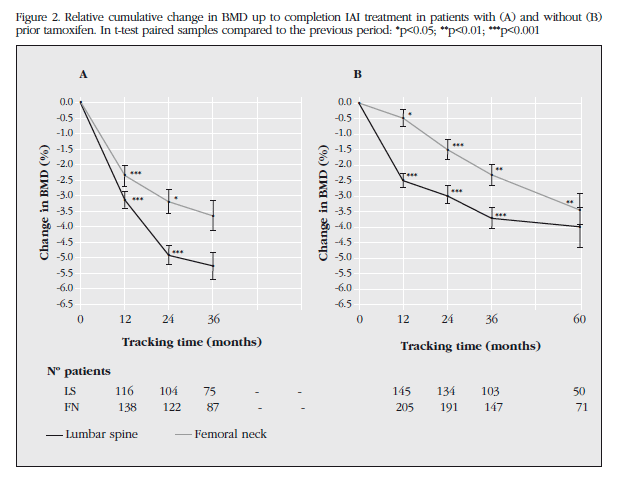
Figure 2 shows the cumulative change in BMD from baseline to the end of treatment. The TMX group showed a more accelerated BMD decrease. So, after 2 years of treatment, patients lost TMX 60% in LS (p<0.001) and 46% in FN (p<0.001) than patients NO-TMX. These differences are maximum 3 years, at which time the TMX patients completed treatment, having lost 70% in LS (p<0.05) and 63% in FN (p<0.01) compared to group NO-TMX after 3 years of therapy.However, the TMX group experienced a decrease in BMD of 5.28% in LS and FN 3.66% in the end of treatment (3 years). For its part, the BMD TMX individuals NO-LS were reduced by 3.99% and 3.43% in FN after 5 years AI therapy. No significant differences were detected in BMD loss at the end of treatment.
AIBL genetic association after treatment
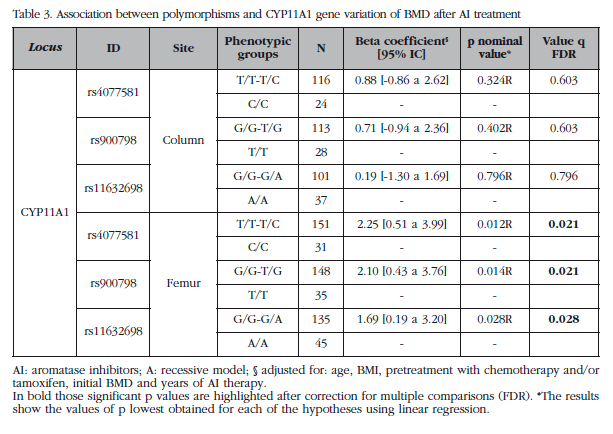
All polymorphisms were genotyped in the Hardy-Weinberg equilibrium. Genotyping efficiency was higher than 97%. Table 3 shows the results of analysis of association of polymorphisms of YP11A1 gene with cumulative BMD loss in FN and LS at the end of treatment. After the FDR correction significant results were obtained for the 3 polymorphisms the CYP11A1 gene with AIBL of CF (q<0.02). No significant results were obtained for LS.
Discussion
This prospective study provides information about the variation in BMD during AI treatment in patients with breast cancer in general clinical practice. The results show that BMD decrease is more accelerated in patients who have received prior therapy with tamoxifen but no significant differences were detected after treatment with respect to those who only received AI. Furthermore, previously a large variability in BMD loss was shown in response to AI treatment [11]. In this study a statistically significant association was detected between decreased BMD at the end of AI treatment and some polymorphisms of the CYP11A1 gene.
Regarding patients who had received prior therapy with tamoxifen, more marked differences in decreased BMD appear at 3 years treatment, in the TMX group lost 70% more in LS and 63% in FN. Tamoxifen acts as an antagonist competitive estrogen receptor in breast tissue, but, in turn, has partial agonist actions in other tissues, such as bone. There is evidence of its beneficial effects in reducing resorption and stimulation of bone formation in postmenopausal women with breast cancer [15]. However, this analysis concurs with some studies indicating that prior tamoxifen therapy considerably increases the effects of AI in bone remodeling, resulting in a further decrease in BMD [16]. One possible explanation for this phenomenon is the "rebound" effect, that is, the positive influence of tamoxifen not only ceases to finish its therapy [16], but also causes a marked reduction in BMD when AI changes. Thus, they increase their resorptive osteoclast action after inhibited state. Tamoxifen is the preferred peri-menopause treatment for women [5]. This would explain the difference observed both in age and initial BMD FN patients with and without previous TMX.
Despite the above, after 5 years of AI therapy, the NO-TMX group was equal to the final BMD loss after 3 years of TMX group [-3.66% vs -5.28% in LS; (P=0.1) and -3.43% vs -3.99% in FN; (P=0.7)], so that no statistically significant differences were detected between groups in BMD values after treatment. It is noteworthy that the rates of BMD loss in LS were at all times higher than FN. In this regard, it is known that trabecular bone is weaker than the cortical in response to AI therapy [17].
Overall, the patients in the cohort B-ABLE lose less BMD compared to previously reported by FFS. For example, the ATAC [18] trial reported losses of 6.08% and 7.24% LS total hip patients treated with anastrozole for 5 years. Patients without bisphosphonates the ABCSG-12 [8] trial suffered losses of 7.8% and 4.1% in LS and FN, respectively, at the end of treatment, even registering decreases of 13.6% in LS at 2 years and 7.3% in FN at 3 years. The MA-17 [7] study, meanwhile, analyzed patients who received tamoxifen before describing loss at 2 years of 5.35% in LS and 3.6% in total hip.
Differences in some features, such as initial BMD values, may contribute to this result. In this regard, most RCTs mentioned showed higher BMD values than those observed in our cohort, leading to bias by regression to the mean. Furthermore, we have found that the prevalence of vitamin D deficiency among Cohort B-ABLE patients is 88.1% [12] at the time of initiating therapy with AI, regardless of the season, which would explain in part the low BMD values. Cohort B-ABLE is subject to a strict evaluation not only of BMD but also the levels of vitamin D and calcium. Vitamin D status has been linked to BMD [19], and most trials have shown that vitamin D supplements are protective against fractures [20] and falls [21]. The patients in our study received supplemental vitamin D in much higher quantities than recommended by the IOM (Institute of Medicine), so that after 3 months of supplements improving levels of 25 (OH) D were achieved, preventing further bone loss [22].
In the present study, an association between BMD loss after AI treatment and polymorphisms in the CYP11A1 gene was detected. The CYP11A1 gene encoding the side chain cleavage enzyme of cholesterol (Alternative: P450scc) that catalyzes the first step and limits steroidogenesis, converting cholesterol to pregnenolone. In addition to cholesterol, may also P450scc hydroxylating vitamin D2, D3 and precursors [23-25], suggesting a broad spectrum of functions in metabolism cell. This enzyme is a mitochondrial membrane bound protein expressed mainly in adrenal cortex, ovary, testes, and placenta [26]. In addition, its expression has also been shown at the RNA level and protein in bone tissue and in osteoblasts [11], suggesting a role for this enzyme in bone metabolism.
In this study, polymorphisms in the CYP11A1 gene: rs4077581 (in the promoter region), rs11632698 (in intron 2) and rs900798 (in the 3 'UTR) were associated with BMD loss at the femoral neck after AI treatment. A statistically significant association was not observed with spinal BMD loss. All DXA study images were carefully analyzed to exclude those devices and/or structural changes (such as osteophytes) that might lead to false elevations in BMD. This procedure has consequences for all spinal results, since degenerative changes in this region can significantly increase BMD. Consequently, the number of patients for this determination was reduced which could explain the lack of statistical significance obtained in LS. In this regard, in a study prior to our group, a similar trend was observed associating these polymorphisms with BMD loss at 2 years of treatment, nominally obtaining significant results for spinal BMD [11].
CYP11A1 gene variants may alter the expression or activity, determining the levels of sex hormones in a tissue, and therefore, be responsible for different phenotypes. This hypothesis would be supported by the fact that other polymorphic variants in this gene have been previously associated with the susceptibility of endometrium [28] and breast cancer [27] as well as to polycystic ovary syndrome [29].
Thus, CYP11A1 activity may play a central role in local synthesis of steroid hormones, being partly responsible for AIBL. Our study has several limitations. First, evaluation of adherence to AI and tamoxifen was found only by a direct question made by the doctor. Second, the exclusion of patients receiving bisphosphonate treatment provides a selection of women with healthier bone, possibly causing a bias in the results. Third, the loss of patients during follow-up causes a decrease beyond 3 years of treatment. However, the study design is closer to the conditions of routine clinical observation. In addition, the implementation of a specific protocol of management of bone health in these patients showed better results in routine oncology practice.
In conclusion, in the CYP11A1 gene polymorphisms are associated with BMD response to treatment with AI. In our opinion, the study of B-ABLE cohort to conclude that the specific control and bone health treatment with calcium and vitamin D in all patients are interventions required during AI therapy, as they have an influence on direct changes in BMD and probably also translate into decreased risk of fragility fracture.
Competing interests
The authors declare no conflict of interest regarding this work.
![]() Correspondence:
Correspondence:
María Rodríguez Sanz
C/ Dr. Aigüader, 88
08003 Barcelona (España)
Correo electrónico:
mrodriguez5@imim.es
Date of receipt: 23/09/2015
Date of acceptance: 06/11/2015
Bibliogragraphy
1. Howell A, Cuzick J, Baum M, Buzdar A, Dowsett M, Forbes JF, et al. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years' adjuvant treatment for breast cancer. Lancet 2005;365:60-2. [ Links ]
2. Geisler J, Helle H, Ekse D, Duong NK, Evans DB, Nordbo Y, et al. Letrozole is superior to anastrozole in suppressing breast cancer tissue and plasma estrogen levels. Clin Cancer Res 2008;14:6330-5. [ Links ]
3. Simpson ER, Mahendroo MS, Means GD, Kilgore MW, Hinshelwood MM, Graham-Lorence S, et al. Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis. Endocr Rev 1994;15:342-55. [ Links ]
4. Winer EP, Hudis C, Burstein HJ, Wolff AC, Pritchard KI, Ingle JN, et al. American Society of Clinical Oncology technology assessment on the use of aromatase inhibitors as adjuvant therapy for postmenopausal women with hormone receptor-positive breast cancer: status report 2004. J Clin Oncol 2005;23:619-29. [ Links ]
5. Burstein HJ, Temin S, Anderson H, Buchholz TA, Davidson NE, Gelmon KE, et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: American Society of Clinical Oncology Clinical Practice Guideline Focused Update. J Clin Oncol 2014;32:2255-69. [ Links ]
6. DeCensi A, Sun Z, Guerrieri-Gonzaga A, Thürlimann B, McIntosh C, Tondini C, et al. Bone mineral density and circulating biomarkers in the BIG 1-98 trial comparing adjuvant letrozole, tamoxifen and their sequences. Breast Cancer Res Treat 2014;144:321-9. [ Links ]
7. Perez EA, Josse RG, Pritchard KI, Ingle JN, Martino S, Findlay BP, et al. Effect of letrozole versus placebo on bone mineral density in women with primary breast cancer completing 5 or more years of adjuvant tamoxifen: A Companion Study to NCIC CTG MA.17. J Clin Oncol 2006;24:3629-35. [ Links ]
8. Gnant M, Mlineritsch B, Luschin-Ebengreuth G, Kainberger F, Kässmann H, Piswanger-Sölkner JC, et al. Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 5-year follow-up of the ABCSG-12 bone-mineral density substudy. Lancet Oncol 2008;9:840-9. [ Links ]
9. Bouvard B, Soulie P, Hoppe E, Georgin-Mege M, Royer M, Mesgouez-Nebout N, et al. Fracture incidence after 3 years of aromatase inhibitor therapy. Ann Oncol 2014. [ Links ]
10. Hadji P, Body JJ, Aapro MS, Brufsky A, Coleman RE, Guise T, et al. Practical guidance for the management of aromatase inhibitor-associated bone loss. Ann Oncol 2008;19:1407-16. [ Links ]
11. Rodriguez-Sanz M, Garcia-Giralt N, Prieto-Alhambra D, Servitja S, Balcells S, Pecorelli R, et al. CYP11A1 expression in bone is associated with aromatase inhibitor-related bone loss. J Mol Endocrinol 2015;55:69-79. [ Links ]
12. Nogues X, Servitja S, Pena MJ, Prieto-Alhambra D, Nadal R, Mellibovsky L, et al. Vitamin D deficiency and bone mineral density in postmenopausal women receiving aromatase inhibitors for early breast cancer. Maturitas 2010;66:291-7. [ Links ]
13. Blake G, Adams JE, Bishop N. DXA in adults and children. In: Clifford J. Rosen, editor. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. 8th ed. Hoboken, New Jersey: John Wiley & Sons, Inc; 2013.p.256. [ Links ]
14. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Statist Soc Ser B (Methodological) 1995;57:289-300. [ Links ]
15. Resch A, Biber E, Seifert M, Resch H. Evidence that tamoxifen preserves bone density in late postmenopausal women with breast cancer. Acta Oncol 1998;37:661-4. [ Links ]
16. McCaig FM, Renshaw L, Williams L, Young O, Murray J, Macaskill EJ, et al. A study of the effects of the aromatase inhibitors anastrozole and letrozole on bone metabolism in postmenopausal women with estrogen receptor-positive breast cancer. Breast Cancer Res Treat 2010;119:643-51. [ Links ]
17. Servitja S, Nogues X, Prieto-Alhambra D, Martinez-Garcia M, Garrigos L, Pena MJ, et al. Bone health in a prospective cohort of postmenopausal women receiving aromatase inhibitors for early breast cancer. Breast 2012;21:95-101. [ Links ]
18. Eastell R, Adams JE, Coleman RE, Howell A, Hannon RA, Cuzick J, et al. Effect of anastrozole on bone mineral density: 5-year results from the anastrozole, tamoxifen, alone or in combination trial 18233230. J Clin Oncol 2008;26:1051-7. [ Links ]
19. Bischoff-Ferrari HA, Kiel DP, Dawson-Hughes B, Orav JE, Li R, Spiegelman D, et al. Dietary calcium and serum 25-hydroxyvitamin D status in relation to BMD among U.S. adults. J Bone Miner Res 2009;24:935-42. [ Links ]
20. Bischoff-Ferrari HA, Willett WC, Wong JB, Stuck AE, Staehelin HB, Orav EJ, et al. Prevention of nonvertebral fractures with oral vitamin D and dose dependency: a meta-analysis of randomized controlled trials. Arch Intern Med 2009;169:551-61. [ Links ]
21. Sanders KM, Stuart AL, Williamson EJ, Simpson JA, Kotowicz MA, Young D, et al. Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA 2010; 303:1815-22. [ Links ]
22. Prieto-Alhambra D, Servitja S, Javaid MK, Garrigos L, Arden NK, Cooper C, et al. Vitamin D threshold to prevent aromatase inhibitor-related bone loss: the B-ABLE prospective cohort study. Breast Cancer Res Treat 2012;133:1159-67. [ Links ]
23. Nguyen MN, Slominski A, Li W, Ng YR, Tuckey RC. Metabolism of vitamin d2 to 17,20,24-trihydroxyvitamin d2 by cytochrome p450scc (CYP11A1). Drug Metab Dispos 2009;37:761-7. [ Links ]
24. Slominski A, Semak I, Wortsman J, Zjawiony J, Li W, Zbytek B, et al. An alternative pathway of vitamin D metabolism. Cytochrome P450scc (CYP11A1)-mediated conversion to 20-hydroxyvitamin D2 and 17,20-dihydroxyvitamin D2. FEBS J 2006;273:2891-901. [ Links ]
25. Tuckey RC, Li W, Zjawiony JK, Zmijewski MA, Nguyen MN, Sweatman T, et al. Pathways and products for the metabolism of vitamin D3 by cytochrome P450scc. FEBS J 2008;275:2585-96. [ Links ]
26. Payne AH, Hales DB. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr Rev 2004;25:947-70. [ Links ]
27. Zheng W, Gao YT, Shu XO, Wen W, Cai Q, Dai Q, et al. Population-based case-control study of CYP11A gene polymorphism and breast cancer risk. Cancer Epidemiol Biomarkers Prev 2004;13:709-14. [ Links ]
28. Terry K, McGrath M, Lee IM, Buring J, De Vivo I. Genetic variation in CYP11A1 and StAR in relation to endometrial cancer risk. Gynecol Oncol 2010;117:255-9. [ Links ]
29. Gao GH, Cao YX, Yi L, Wei ZL, Xu YP, Yang C. Polymorphism of CYP11A1 gene in Chinese patients with polycystic ovarian syndrome. Zhonghua Fu Chan Ke Za Zhi 2010;45:191-6. [ Links ]











 texto en
texto en