INTRODUCTION
Breast cancer represents a major scientific, clinical, and societal problem. It is the most common malignancy and the second leading cause of cancer death in females following lung cancer [1, 2] with more than a million new cases and 370,000 deaths yearly worldwide [3]. In many developing countries, the incidence of breast cancer is now rising sharply due to changes in reproductive factors, lifestyle, and increased life expectancy [4]. The invasive ductal carcinoma is the most common type of breast cancer and comprises 70% to 80% of all cases. It starts in a milk duct of the breast, breaks through the wall of the duct, and grows into the fatty tissue of the breast, that it may be able to spread (metastasize) to other parts of the body through the lymphatic system and bloodstream [5]. The cells invasion must occur from detached and penetrate the basement membrane; adherent cells lose their normal attachment to the substrate [6]. Proliferation has higher sensitivity to the extracellular matrix, which maintains an actively proliferating phenotype in the newly encountered matrix [7]. The ductal carcinoma is an ill-defined mass, sometimes adherent to the skin or underlying muscle. The tumor is of varying size and may be associated with calcification. The histomorphology of the tumor is highly variable, ranging from a low-grade tumor showing mildly pleomorphic tumor cells arranged in tubules with little mitotic activity to a high-grade tumor showing highly pleomorphic tumor morphology, with the tumor cells arranged in solid sheets and groups, brisk mitotic activity and abundant tumor necrosis [8]. The Nuclear morphology is evaluated in the variation of nuclear size, the regularity of the nuclear border, hyperchromasia, and prominence of the nucleolus. Also, it has been reported that histologic grade for breast cancer remains a prognostic factor despite changes in the tumor size and the number of positive lymph nodes [9]. The histological grade is commonly employed and has been shown to be of independent prognostic significance. The studies showed a very strong correlation with prognosis; patients with grade I tumors havesignificantly better survival than those with grade II and III tumors (p < 0.0001) [10].
Progranulin is 88-kDa glycoprotein and known as GP88, PC-cell derived growth factor or acrogranin,GP88 gene is located on the 21q portion of chromosome 17, while the mouse gene was found on chromosome 11 [11]. The autocrine growth factor GP88 is abundantly expressed in epithelial cells, immune cells, neurons, and chondrocytes [11]. Also, involved in a variety of biological processes, including embryogenesis, inflammation [12], wound healing [13], host defense [14], and cartilage development and degradation [15]. The importance of the Progranulin (GP88) is no unique receptor for GP88 has been identified [16], it has significant biological effects in different types of cancer for stimulating cell proliferation, migration, invasion, angiogenesis, malignant transformation, resistance to anticancer drugs, and immune evasion [17]. GP88 has been shown to bind the membrane proteins sortilin and tumor necrosis factor receptors (TNFR) 1 & 2 [18]. It is thought to exert its mitogenic effect through the stimulation of both the mitogen-activated protein kinase (MAPK) and the phosphatidylinositol 3-kinase (PI3K) pathways [19]. GP88 stimulates the expression of the angiogenic growth factor (VEGF) in breast cancer cell lines; breast cancers with elevated GP88 levels have higher VEGF levels and microvascular density [20]. GP88 is an important marker in breast cancer; it can be measured in healthy and breast cancer patients [21]. Increased secretion of GP88 is associated with more aggressive forms of breast, brain, renal, cervical, and other tumors [12].
HER-2/neu (also called c-erbB-2) is a proto-oncogene located on chromosome 17 and encodes a 185-kDtransmembrane glycoprotein receptor (p185 HER2) which has a tyrosine kinase activity and related to the epidermal growth factor receptor (EGFR). It is amplified or overexpressed in about 25% to 30% of human breast cancer, which is associated with tumor aggressiveness [11], and maybe related to variability in the interpretation of protein expression levels [22]. The predictive value of HER-2/neu status with respect to response to therapy as hormonal therapy, resistance to alkylating agent-based chemotherapy, and taxanes [23, 24]. So, the aim of the present work was designed to study the Immunohistochemical expression of the GP88 as a novel prognostic biomarker in human invasive breast carcinoma.
METHODS
SAMPLE PREPARATION
Formalin-fixed and paraffin-embedded of 100 prospective human breast specimens from women's have breast lesion. The samples were diagnosed and differentiated into sixty samples of invasive ductal carcinoma (IDC), thirty samples of benign lesion, and ten samples from normal areas adjacent to the benign tumors were included. Tissue samples were obtained from the Department of Pathology, Medical Research Institute (MRI), Alexandria University, Egypt, All individual cases were asked to freely volunteer for the study and informed written consents were gathered prior to their inclusion in the study according to the guide ethics of institute MRI (IORG#: IORG0008812). Samples from all studied cases were subjected to the following:
Clinical parameters: The patients' age, tumor size, and lymph node metastasis (LNM) status were collected from data sheets from pathology department.
Histopathological Examination: Paraffin-sections were picked up for each sample on glass slides. The slides were stained by hematoxylin and eosin (H&E) stains in the Histochemistry and Cell Biology department and reviewed by two pathologists (Diagnosis of the specimens was made according to the WHO classification of the Tumors) [25]. The samples were diagnosed and classified the invasive ductal carcinoma into grades (grade I (IDC GI), grade II (IDC GII), and grade III (IDC GIII)).
Routine Biological Markers of Estrogen and Progesterone Receptor: The result of the Immunohistochemical staining of ER and PR. were examined under the light microscopy and semiqualitative positivity of degree was evaluated in pathology department
IMMUNOHISTOCHEMICAL TECHNIQUE
The detection of both GP88 and HER2/neu protein used avidin-biotin complex protocol (ABC) and DAB stain [26]; In brief; paraffin-embedded specimens were cut into 5µm sections and picked up on the coated slides. The sections were deparaffinized on two changes of xylene and rehydrated in alcohol. The slides were submerged in antigen retrieval (citrate buffer saline pH 6) in an oven at 95°C for 20 minutes, and then left at room temperature for 20 minutes to cool. They were treated with 3% H2O2in PBS to quench the endogenous peroxidase activity, followed by incubation with serum blocking reagent for 30 minutes to block nonspecific binding. The slides were incubated with primary antibody for GP88 (Biorbyt Company®, London, UK) and another one in HER2/neu (Novus Biologicals Company®, USA) at 4ºC overnight. The slides were treated with conjugated secondary antibody (ABC-HRP reagent) for 30 minutes, and then they were washed, stained with diaminobenzidine (DAB), and counterstained with hematoxylin. The antibody of negative control slide was replaced with PBS. Each step was followed by PBS washing. Immunohistochemical evaluation of results was arbitrarily graded as negative (0), weak (+1), moderate (+2), and strong (+3).
IMAGE ANALYSIS
The Integrated Optical Density (IOD) of GP88 and HER2/neu in normal, benign, and IDC groups were analyzed using a digital image analyzer. Images were photographed and recorded using an Olympus microscope equipped with spot digital camera and image J software. The maximum and minimum pixel recorded the integrity of intensity color based on Gray-level acquisition. The analysis of data was carried out by reading ten images for each case. The mean value of each reaction was based on the mean of the pixel number [27]. IOD based on Gray-level transition probabilities in digitized images were graded from dark to light (0 up to 250). The average score across the whole images should be taken; IOD in digitized images were calibrated from strong to weak (180 down to 70) by pixel. This calculation was proceeding after subtracting the pixel value from 250, the pick of lighter elimination.
All data were normally distributed according to the Kolmogorov-Smirnov (K-S) normality test, and then analyzed using statistical software package SPSS 20. P values ≤ 0.05 were considered statistically significant.
RESULTS
HISTOPATHOLOGICAL INVESTIGATION
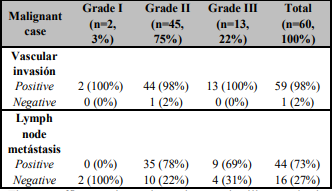
The histopathology diagnosis of the studying cases found the benign cases revealed as the majority (83%, 25/30) of fibroadenoma, while 17% (5/30) cases were represented with fibrocystic disease. At malignant cases, there was a majority of invasive ductal carcinoma (IDC) (98%, 59/60), while 2% (1/50) of the malignant cases were represented with invasive lobular carcinoma (ILC). In addition, according to Scarf'-Bloom-Richardson histological grading system of breast cancer, the majority of the malignant cases diagnosed as grade II (45/60, 75%), grade III cases were represented by 22% (13/60), and the minority cases was grade I (2/60, 3%) of the malignant cases as in the (Table 1).
The paraffin section photomicrograph illustrated the histopathological changes of the study groups. The normal breast tissue showed acini lined by epithelial cells, an intact myoepithelial cell layer attached to the basement membrane of acini (Figure 1A). A mild adenosis and cyst formation with mild epithelial hyperplasia in the adjacent duct and intact myoepithelial cell layer in fibrocystic disease of the benign breast case (Figure 1B). The tumor cells of invasive ductal carcinoma cases have abundant eosinophilic cytoplasm and large moderately pleomorphic round to ovoid vesicular nuclei arranged in cords and some contain small nucleoli. Infiltrated tumor cells in desmoplasticstroma represented GII (Figure1C). The neoplastic growth formed of nests and tubules and hyperchromatic nuclei and largely pleomorphic vesicular nuclei were exhibiting mitotic activity in ductal epithelial cells with many pyknotic cells, as well as, many residual nuclei were seen in GIII (Figure 1D).
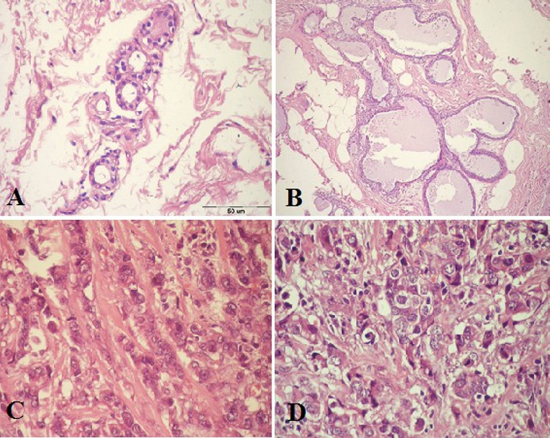
Figure 1. Paraffin section photomicrograph stained by (H&E stains, Bar=50 µm).A:- Note the acini present in a normal lobule with dark-staining epithelium cells nuclei and homogenous pink cytoplasm and the underlying myoepithelial cells with clear cytoplasm, wild and edema connective tissue a]was seen. B: A fibrocystic case of benign lesion with apocrine cyst formation and mild epithelial hyperplasia in the adjacent duct and intact myoepithelial cell layer. C: IDC grade II case, show tumor cells have abundant eosinophlic cytoplasm and large moderately pleomorphic round to ovoid vesicular nuclei arranged in cords and some contain small nucleoli. There are tumor cells infiltrated by desmoplasticstroma. D: IDC case grade III, show solid nests of tumor cells with large pleomorphic nuclei and some prominent nucleoli. There are numerous mitotic figures, large vesiculated nuclei and many residual nuclei and vacuolated cytoplasm.
CLINICAL PATHOLOGICAL PARAMETERS
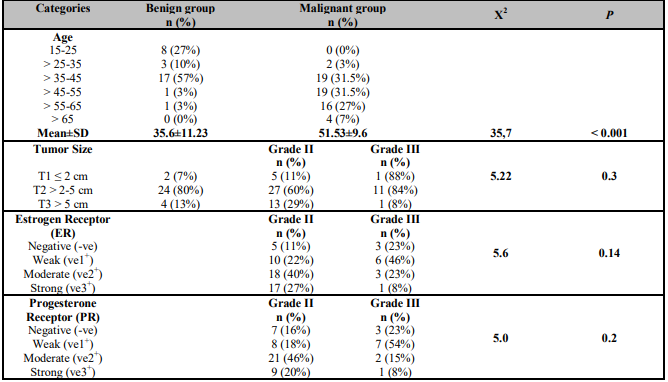
The age ranges of benign and malignant groups were 15-64 and 15-74 years with a mean ± SD of 36 ± 11 and 52 ± 10 years respectively. The data reported of the benign group cases 57% (17/30) at age range > 35-45 years versus of the malignant group cases 31.5% (19/60) at age ranges > 35-45 and > 45-55 years respectively. Neither of the benign cases was above 65 years nor of malignant cases under 25 years as shown in (Table 2).
According to the tumor size assessed according to TNM (Tumor, Node, Metastasis) staging system of breast cancer, there was 80% (24/30) of the benign cases was T2 (> 2-5 cm) versus 66% (27/45 grade II and 11/13 grade III) of the malignant cases were T2 (> 2-5 cm) as illustrated in the (Table 2). Whereas the percentage of positive vascular invasion (VI) was 98% (59/60) were distributed in the (Table 1), as well as, Lymph Node Metastasis (LNM) was illustrated in the (Table 1). Also, Noticed the majority of positive cases were represented (44/60.3%) and free of lymph node metastasis cases were 16/60 (27%).
IMMUNOHISTOCHEMICAL STAINING INVESTIGATION
The immunostaining expression of human epidermal growth factor receptor-2 (HER2/neu) was detected as diffuse brown color detected in the cell membrane of the ductal epithelial cells. There was a weak expression in the normal ductal epithelial cells of the control breast tissue (Figure 2A). HER2/neu was expressed as a moderate expression in the few proliferating ductal epithelial cells of the Benign cases less than ten cells of the field (Figure 2B). Whereas at the malignant cases an increase of the immunostaining of the HER2/neu expression was ranged from moderate to strong. A strong positivity expression (+3) showed mostly in the epithelial cell membrane > 50 of IDC grade III (Figure 2C) and a Moderate positive expression (+2) of HER2/neu in the ductal epithelial cells membrane with dark brown stain showed in IDC grade II (Figure 2D).
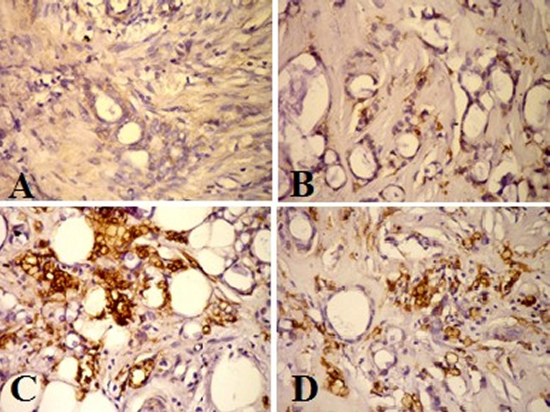
Figure 2. HER2/neu Immunohistochemical staining photomicrograph of breast paraffin section used ABC protocol (DAB stain, Bar = 50 µm) show:A: Control breast tissue very weak immunoactive at epithelial ductal cells. B: Benign case with mild expression of HER2/neu in the cell membrane of the ductal epithelial cell <10 cells. C: IDC grade II, strong positive immunostaining of HER2/neu (+3) in the epithelial cell membrane > 50. D: Moderate positive expression (+2) of HER2/neu in the ductal epithelia cells membrane >30.
The immunostaining expression of GP88 was detected as diffuse, homogenous brown color stained the cytoplasm and membrane of the ductal epithelial cells of the breast lesions studies group. GP88 Immunoexpression showed a negative expression in normal breast tissue, as well as in the benign case beside a weak expression in stromal tissue (Figure 3 A and B). The high expression of the GP88 was shown in the malignant cases group. There was strong immunostaining of GP88 (+3) in the epithelial cell and marked positive expression (+2) of GP88 in the cytoplasm of the ductal epithelial cells in the IDC grade II (Figure 3 C and D). Also, the IDC grade III showed strong immunoexpression (+3) of GP88 which appeared as the homogenous expression in the invasive ductal epithelial cell and surrounding stromal tissue, in another cases there was coarse and homogenate immunostaining at the epithelial ductal cell and stroma (Figure 3 E and F).
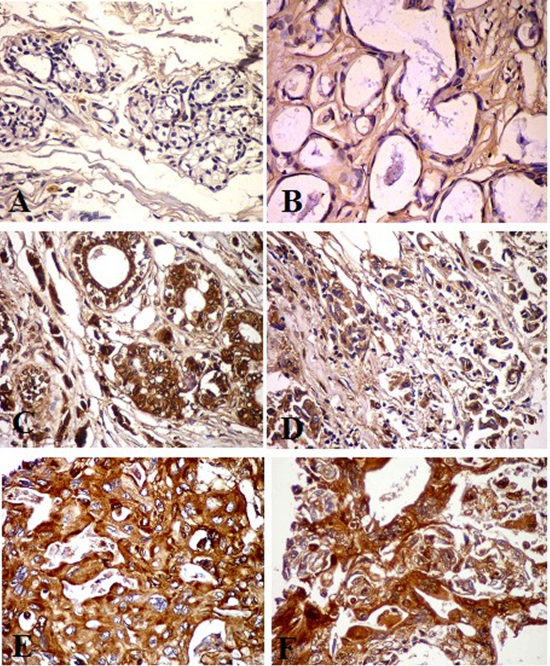
Figure 3. GP88 Immunohistochemical staining photomicrograph of breast paraffin section used ABC protocol (DAB stain, Bar =50 µm) show:A: Control breast tissue. B: Benign case with negative expression of GP88 in the cytoplasm of the ductal epithelial cell and weak expression in stromal tissue. C and D: Invasive ductal carcinoma (IDC) grade II, strong immunostaining of GP88 (ve3+) in the epithelial cell at the luminal surface. D: Marked positive expression (ve2+) of GP88 in the cytoplasm of the ductal epithelia cells. E and F: IDC grade III, note strong expression (ve3+) of GP88 as the homogenous expression of GP88 in the ductal epithelial cell and surrounding stromal tissue. F: High expression of the GP88 with coarse and homogenate immunostaing at the upper surface of the epithelial ductal cell.
Semiquantitative Immunohistochemical Evaluation
Hormonal status of both estrogen and progesterone receptor (ER&PR) used as a biological marker in the malignant cases were differentiated to malignant grade II & III and illustrated in (Table 2) respectively. According to human epidermal growth factor receptor-2 (HER2/neu) expression, noticed that 53% (24/45) of IDC grade II were weakly positive (-ve), while 38% (5/13) of IDC grade III were strongly positive (ve3+), as shown in (Table 3). As regard to GP88 immunostaining expression resulted a moderate positive expression (ve2+) in 71% (32/45) of grade II IDC and strong (ve3+) in 77% (10/13) of grade III IDC as illustrated in (Table 4).
Immunohistochemical Integrated Optical Density (IOD)
Figure 4 illustrated a gray value detection of the binary evaluation at the 8-bit image of the stain density was quantitative digitally by pixel in the integrated region of optical density (ROD) as following:
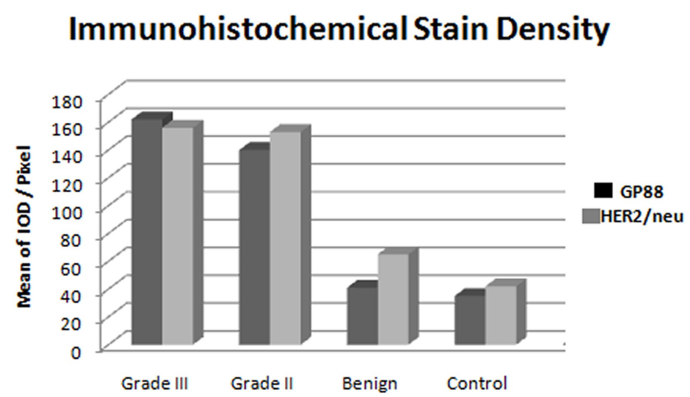
Figure 4. Bar Chart illustrate the mean value of HER/2neu and GP88 IOD of the immunohistochemistry expression in the breast cases study, note a significant increased of both markers in grade II &III of malignant cases compared to the benign and control, while there are insignificant difference between both markers.IOD: Integrated Optical Density.
The mean and SD values of HER2/neu IOD for breast lesions illustrated the different ranges from 42 ± 3.82, 63 ± 5.34, 143 ± 5.49 and 169 ± 4.46 as control, benign and IDC grade II and III were respectively. There was a statistically significant difference between the benign group and malignant one, as well as between the malignant grade II and III of IDC (P < 0.001).
The mean and SD values of GP88 IOD for control, benign, and IDC grade II and III were 35 ± 3.41 ± 4, 140 ± 8, and 162 ± 7 respectively. A significant statistical difference of GP88 IOD was noticed between grade II and grade III of IDC cases (P < 0.001), as well of both control and benign group (P < 0.001).There was no statistical significant difference between GP88 IOD of control and benign groups (P = 0.1).
Correlation between GP88 IOD and Clinicopathological Parameters
There was no statistical significant correlation between the GP88 IOD and patients' age (r = -0.14, P = 0.28), ER status (r = -0.09, P = 0.50), PR status (r= 0.06, P = 0.65) and HER2/neu status (r = 0.22, P = 0.08) of the studied breast cancer cases, while highly statistical significant correlation was noticed between the GP88 IOD and tumor size (r = 0.354**, P = 0.006), tumor grade (r = 0.353**, P = 0.006) and LNM status (r = 0.493**, P = 0.000) as in Table 5.
DISCUSSION
Progranulin or GP88 is an autocrine growth factor and pleiotropic regulatory protein that has been shown to play role in tumorigenesis, including proliferation, survival, migration, angiogenesis invasion and matrix metallo-protease activity [28, 29], as well as, in wound healing and in inflammation in normal tissues [29]. The study was evaluated the role of GP88 through the digital immunostaining density under the correlation with the clinical pathological parameter in the breast cancer lesions.
The study evaluated a statistical significant increase in the immunohistochemical expression of GP88 in IDC, versus, normal tissues and benign tumors (P < 0.001). This result is in alignment with previous finding reported a high level of GP88 expression in breast cancer biopsies versus benign lesions and normal mammary epithelial tissues [30, 31]. The pathological studies with 203 formalin-fixed paraffin-embedded human breast cancer tissue biopsies indicated that GP88 was preferentially expressed in ductal carcinoma with little expression in lobular carcinoma while benign lesions and normal mammary epithelial tissues were negative [32]. In addition, circulating GP88 level in the serum of breast cancer patients was increased in compared to healthy volunteers [28]. Moreover, the parental SW13 cells (cell derive from an adrenal carcinoma) poorly tumorigenic in vivo; however, by over-producing GP88, they become highly tumorigenic in mice [33, 34]. This finding revealed that the SW13 cells are highly sensitive to the proliferative effects of GP88. So, the digital analysis (IOD) of the GP88 in the present work revealed the role of the GP88 expression associated with the increased tumor grade in the breast cancer and detected its role in the proliferation and invasive of the tumor cell in breast tissue. The immunostaining positivity of GP88 was expressed from marked to intensity in the breast malignant lesion and differentiated in both grade II and grade III, this results were indeed the role of the GP88 expression induced and associated with division and invasive tumor cells. This finding was confirmed that the pgrn (GP88) stimulate cell division, invasion, and survival demonstrates [33]. Also, it has been revealed the role of GP88 expression for regulates multiple processes or stages in carcinoma progression [35].
However, there was no statistically significant correlation between immunohistochemial expression of GP88 and patients' age (r= -0.1, P = 0.3). This result is consistent with the results of other previous studies [29, 36], who reported the clinical and tumor characteristics (p-value risk ratio 95% ) confidence interval lower upper age in years Age ≤ 50 compared to > 50. Therefore, it could be agreed that the tissue GP88 level is predictive of recurrence in patients with hormone-responsive BC [37], while the patients with early-stage BC would be suggested that serum GP88 determination has the potential to have additional clinical utility. Arechavaleta-Velasco et al, revealed that the PGRN is a unique secretory glycoprotein and the focus of its prospective use as a biomarker or as a therapeutic target in cancer [17].
The large tumor size, high tumor grade, and positive lymph nodes are all signs of poor prognosis and elevated GP88 expression have shown to be associated with tumorigenesis and poor prognosis in several cancer types including ovarian [31], renal [38], prostate [39], liver [40], esophageal [41, 42] and breast cancers [42]. The current study showed a highly statistically significant correlation between the immunohistochemical expression of GP88 and tumor size (r= 0.354, P= 0.006) of malignant breast cases. This result is going in accordance with some authors [36], but was contrasted with other [30, 43]. Moreover, a highly statistically significant correlation was observed in the current study between GP88's immunohistochemical expression and tumor histological grade (r= 0.353, P= 0.006). This result is agreement to the overexpression of GP88 in 80% invasive ductal carcinoma, where it correlated with the clinical variables of poor prognosis such as tumor grade, p53 expression and Ki67 index [32, 36]. Immunohistochemical expression of GP88 in the present study exhibited a highly statistically significant correlation with LNM of the studied breast cancer cases (r= 0.493, P = 0.000). A previous study found that high levels of GP88 expression were closely correlated with clinicopathological parameters such as lymph node metastasis [36]. The histopathological features are independent predictors of clinical outcomes such as lymph node status, tumor size and tumor grade for invasive ductal carcinoma patients were based evidence that IHC analysis of GP88 expression maybe help a predict treatment outcomes in a prognostic role.At the correlation between the GP88 and hormonal status as a routine marker and necessary for the treatment and therapy of BC, our results in IDC patients found no statistical significant correlation between the digital quantitative immunohistochemical expression of GP88 and ER (r= 0.09, P = 0.50) and PR (r= 0.06, P = 0.65) status. These results are conflicted to the result of the GP88 positive staining was common in ER/PR negative samples than that in ER/PR positive tumors [43], also, the agreement to present result, that there was no statistical correlation between GP88 expression and ER and PR levels [29]. However, screening of GP88 expression in human breast cancer cell lines indicated that GP88 was highly expressed in both ER positive and ER negative in human breast carcinomas [44, 45]. This lack of correlation between GP88's immunohistochemical expression and ER status indicated that the growth factor receptors promote proliferation without requiring ER-mediated growth signalling [46].
It has been reported that level of GP88 expression was a major determinant of the intrinsic activity of the mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3'-kinase (PI3K) in cell line studies suggesting that the mitogen-activated protein kinase and phosphatidylinositol 3'-kinase signaling pathways may be involved in the promotion of tumor invasion and migration by GP88 [16, 34]. However, the current study revealed no statistical significant correlation between the immunohistochemical expression of GP88 and HER2/neu status (r = 0.2, P = .08) of the studied breast cancer cases. This result is going in accordance with many studies reported that there was no correlation between expression of GP88 and HER2/neu, and indicated that GP88 and HER2/neu were independent biomarkers [29, 32]. Other investigator found that 25% of HER2-overexpressing biopsies (3+ by IHC) were strongly positive for GP88 [47] and the treatment of HER2-overexpressing cells with Trastuzumab decreased the levels of MAPK phosphorylation [48]. The results are going to that the activation of mitogen-activated protein kinase (MAPK) signaling pathways in breast cancer cells by the over-production of growth factors [43]. So, GP88 can activate MAPK signaling pathway and phosphorylated MAPK regardless of the HER2 expression levels, thereby GP88 is not necessarily dependent on HER2 overexpression, because it has its own ability to activate MAPK pathway [49]. This may be indicated that HER2/neu and GP88 are independent biomarkers.
Finally, the study found that high progranulin expression was associated with higher breast histopathological features, which are strong independent predictors of clinical outcomes such as lymph node status, tumor size and tumor grade for patients with invasive ductal carcinoma, as well as, the digital quantitative immunostaining of GP88(IOD) in IDC cases was evident a high statistically significant correlation compared to the benign and malignant cases as well as between the IDC grade II and grade III (P = 0.0001). Beside of no correlation between GP88 with ER, PR, and HER2/neu expression.
CONCLUSIONS
It may be suggested that the GP88 is involved in processes of cells invasiveness, regulate tumor cell progression and can act as a biomarker along with a therapeutic target. Therefore, the further clinical study on more of breast lesions will be guaranteed to improve the thinkable of GP88 use as diagnosis and treatment.