Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
REC: Interventional Cardiology
versão On-line ISSN 2604-7276versão impressa ISSN 2604-7306
REC Interv Cardiol ES vol.5 no.1 Madrid Jan./Mar. 2023 Epub 18-Mar-2024
https://dx.doi.org/10.24875/recic.m22000343
DEBATE
Debate. Drug-coated balloons for de novo coronary artery lesions. Evidence available and potential superiority in some settings
1Cardiovascular Research Group, Fondazione Ricerca e Innovazione Cardiovascolare, Milán, Italia
SEE RELATED CONTENT:
https://doi.org/10.24875/RECICE.M22000347
QUESTION: What is the evidence available on the use of drug-coated balloons (DCB) in the de novo lesion setting?
ANSWER: The use of DCB to treat de novo lesions is the most compelling argument regarding this technology, an area that has advanced significantly over the last 5 years. Only recently, investigators and companies have begun to understand that this area also needed strong and reliable scientific evidence similar to the one provided for stent platforms, to understand the real safety and efficacy profile of DCB in the de novo lesion setting. Argument here is currently quite strong: the BASKET-SMALL 2 trial (700 patients) showed no differences at 3 years between paclitaxel-coated balloon and drug-eluting stents (DES),1 the EASTBOURNE (2100 patients) showed the 1-year safety and efficacy profile of the first sirolimus-coated balloon (target lesion revascularization, 5%),2 the PICCOLETO II trial3 showed fewer major adverse cardiovascular events with another paclitaxel-coated balloon compared to an everolimus-eluting stent in the small vessel setting at 3-year follow-up. Finally, the RESTORE SVD trial showed similar results with another paclitaxel-coated balloon at the long-term follow up and similar data vs DES.4 Interestingly enough, the long-term follow-up of the 2 latter trials was presented in September 2022 at the TCT Conference (late breaking clinical science session) confirming that this field is currently highly active.
Q.: Do you think there is enough evidence to recommend their use in the routine clinical practice?
A.: Evidence is compelling enough to recommend DCB in this setting. However, some simple rules should be applied a) we recommend using these devices in the in-stent restenosis setting under imaging guidance for proper lesion assessment. Therefore, treatment of small coronary vessels (< 2.5 mm) can be adopted. Afterwards, larger vessels should also be treated with DCB. The important thing here is to “have a good eye” to treat coronary artery dissections left after treatment (please see below); b) class effect does not exist for DCB. Therefore, only devices with robust clinical data should be used in this setting. Angiographic monitorization is often unnecessary unless DCB is used in a complex lesion setting without prior reliable experience or scientific evidence.
Q: Do you think that there are differences in the results obtained from the studies and in the level of evidence according to the size of the target vessel?
A.: We believe that most DCBs can also be used for larger vessels (> 3 mm), but a wide use in this setting can be suggested in selected cases only where the stent is not seen as a safe enough solution (highly complex calcified lesions, trifurcations…). Also, the broader use of DCB requires more clinical data—that are still pending—which will hopefully be provided within the next 2 to 3 years. Unfortunately, direct comparisons among DCBs are not available yet except for a small, sponsored trial. A couple of years ago we “indirectly” compared a paclitaxel- and a sirolimus-coated balloon in the SIRPAC trial (1100 patients) showing no differences at 1 year regarding hard endpoints.5 The ongoing TRANSFORM I trial, which has recently completed the enrollment, is comparing paclitaxel- and sirolimus-based DCBs on mid-term angiographic and optical coherence tomography outcomes.6 This mechanistic study is important because it will shed light on the current effect of these drugs on the vessel wall, and on the role paclitaxel plays determining late lumen enlargement for a direct effect in the adventitia, something that is still to be proven by sirolimus. Finally, the ongoing TRANSFORM II trial that is comparing a sirolimus-based DCB to a DES will shed light on the long-term role of this technology.7 In this study, whose primary endpoint is TLF, patients with native vessel disease will be treated and followed for 5 years.
Q: In which cases would you consider using DCBs to treat de novo coronary artery lesions?
A.: To be honest, given the drawbacks of stents in the small vessel disease and complex lesion settings, here a DCB would be our first choice due to the inherent safety of this technology. For example, in a heavily calcified coronary lesion, despite proper lesion preparation, we often prefer using a DCB when we are not totally sure that the stent will accommodate perfectly with an adequate expansion and apposition. The takeaway here is that DCBs can also lead to restenosis. However, they are safe and do not lead to thrombosis or acute vessel occlusion. Only in case of flow-limiting dissections or acute recoil, a bailout stent should be used after a DCB. Also, we should always remember that a stent-like result after DCB angioplasty should not be expected or is not needed either.
Q.: What is the predilatation protocol, cross-over criteria, and specific DCB treatment technique in this setting?
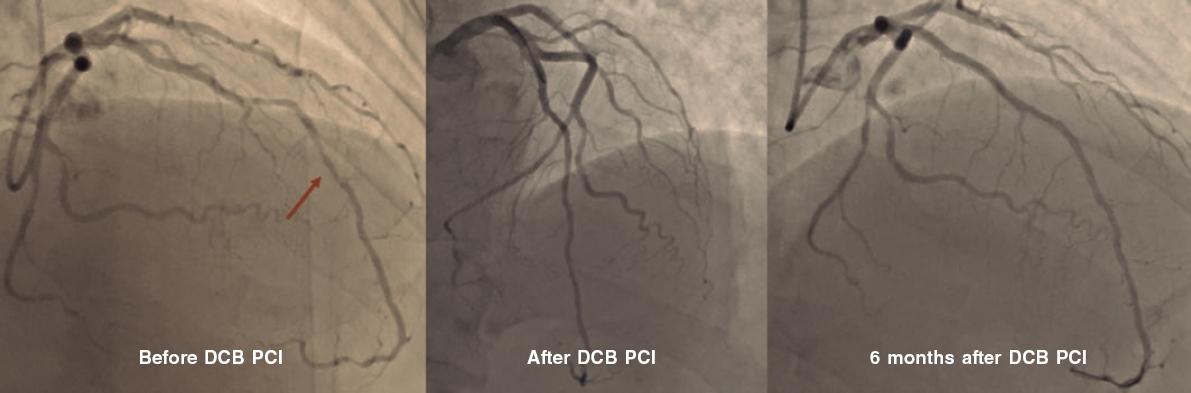
A.: This is a long topic of discussion, and dedicated courses should be followed to “have a good eye” on DCB angioplasty. Our initial suggestion is to adopt a stepwise approach when performing a DCB angioplasty, which means that the main goal is to achieve good results after proper predilatation with whichever tools are available at the cath lab. We can still cross over to a stent angioplasty at any time before drug delivery so make the final choice between DCB and DES after lesion preparation only. Proper predilatation means final stenosis < 30% and no major or flow-limiting dissections.8 After this goal is achieved, the DCB can be used to cover the entire segment treated while keeping inflation for, at least, 30 seconds (possibly 60). In our routine clinical practice we use semi-compliant balloons, and quite often scoring balloons, but other centers use non-compliant balloons as the first choice. The balloon-vessel ratio should often be 1:1, but exceptions exist depending on the target lesion. In the end, type A or B dissections should be sought, and not feared (figure 1). Our group has previously demonstrated how these dissections are safe and not associated with acute vessel occlusions.9 Recently, investigators from Japan have shown how dissections are associated with improved penetration and increased lumen gain at 6 months after paclitaxel-based DCB angioplasty.10 To be considered “expert” DCB users, stenting rate after DCB should be < 10%.
REFERENCES
1. Jeger RV, Farah A, Ohlow MA, et al. Long-term efficacy and safety of drug-coated balloons versus drug-eluting stents for small coronary artery disease (BASKET-SMALL 2):3-year follow-up of a randomised, non-inferiority trial. Lancet. 11 07 2020;396:1504-1510. [ Links ]
2. Cortese B, Testa L, Di Palma G, et al. Clinical performance of a novel sirolimus-coated balloon in coronary artery disease:EASTBOURNE registry. J Cardiovasc Med (Hagerstown). 2021;22:94-100. [ Links ]
3. Cortese B, Di Palma G, Guimaraes MG, et al. Drug-Coated Balloon Versus Drug-Eluting Stent for Small Coronary Vessel Disease:PICCOLETO II Randomized Clinical Trial. JACC Cardiovasc Interv. 2020;13:2840-2849. [ Links ]
4. Tang Y, Qiao S, Su X, et al. Drug-Coated Balloon Versus Drug-Eluting Stent for Small-Vessel Disease:The RESTORE SVD China Randomized Trial. JACC Cardiovasc Interv. 2018;11:2381-2392. [ Links ]
5. Cortese B, Caiazzo G, Di Palma G, De Rosa S. Comparison Between Sirolimus- and Paclitaxel-Coated Balloon for Revascularization of Coronary Arteries:The SIRPAC (SIRolimus-PAClitaxel) Study. Cardiovasc Revasc Med. 2021;28:1-6. [ Links ]
6. Ono M, Kawashima H, Hara H, et al. A Prospective Multicenter Randomized Trial to Assess the Effectiveness of the MagicTouch Sirolimus-Coated Balloon in Small Vessels:Rationale and Design of the TRANSFORM I Trial. Cardiovasc Revasc Med. 2021;25:29-35. [ Links ]
7. Greco A, Sciahbasi A, Abizaid A, et al. Sirolimus-coated balloon versus everolimus-eluting stent in de novo coronary artery disease:Rationale and design of the TRANSFORM II randomized clinical trial. Catheter Cardiovasc Interv. 2022;100(4):544-552. [ Links ]
8. Cortese B. The role of optimal lesion preparation for de-novo coronary vessels when a stentless intervention strategy is planned. Minerva Cardioangiol. 2020;68:51-56. [ Links ]
9. Cortese B, Silva Orrego P, Agostoni P, et al. Effect of Drug-Coated Balloons in Native Coronary Artery Disease Left With a Dissection. JACC Cardiovasc Interv. Dec 2015;8:2003-2009. [ Links ]
10. Yamamoto T, Sawada T, Uzu K, Takaya T, Kawai H, Yasaka Y. Possible mechanism of late lumen enlargement after treatment for de novo coronary lesions with drug-coated balloon. Int J Cardiol. 2020;321:30-37. [ Links ]











 texto em
texto em