INTRODUCTION
NOD.CB17-Prkdcscid/J mice, commonly known as NOD-SCID mice, are a genetically engineered strain of mice frequently used in biomedical research (1). These mice are characterized by a severely compromised immune system, due to a specific mutation in the Prkdc gene, which encodes a protein kinase vital for DNA repair during immune cell development, and by their background strain, the nonobese diabetic (NOD) mouse, which provides additional unique immune system characteristics. Due to the mutation in the Prkdc gene, these mice lack functional T cells and B cells, which makes them unable to mount effective adaptive immune responses (2). This deficiency prevents them from rejecting materials from foreign species, such as human cells. The NOD background contributes to defects in the natural killer (NK) cell function, which is typically less compromised in other SCID strains (2,3). This makes NOD-SCID mice particularly useful for xenograft experiments, in which human cells or tissues are grafted into mice. Therefore, they are extensively used in research involving the transplantation of human cells (1-4).
In the early 2000s, physicians started observing osteonecrosis of the jaw (ONJ) in patients without a history of radiation exposure and realized that most of these patients had breast cancer metastatic to bone or myelomatous disease and were on bisphosphonate (BP) therapy. This led investigators to propose an association between BP use and ONJ (5). The estimated incidence is 1-90 cases per 100 000 patients on BPs per year (6). Most of these cases occur in patients on high doses of BPs (especially zoledronate and pamidronate) due to cancer. Treating ONJ may be rather difficult. In recent years, mesenchymal stem cells (MSCs) have been proposed as candidates for cellular therapies in different conditions including ONJ. MSCs are adult multipotent stromal cells that can differentiate into a variety of cell types, such as osteoblasts, chondrocytes and adipocytes (7,8). Given their ability to differentiate into osteoblasts, they are attractive candidates for bone regeneration therapies. Recent research underscores the multifaceted role of MSCs, mediated not only by differentiation into osteoblasts, but also by the modulation of the bone-healing environment through paracrine effects, involving the secretion of growth factors and cytokines (9-11). Therefore, some studies in animal models have obtained promising results with MSCs applied by either systemic or local routes (12-15). To explore the potentially beneficial effect of a systemic administration of human MSCs in vivo, we tried to develop a murine model of ONJ and ovariectomy (OVX)-induced osteoporosis. To prevent rejection, we used immunodeficient mice and protocols previously applied to induce ONJ and osteoporosis in immunocompetent mice.
MATERIAL AND METHODS
NOD.CB17-Prkdcscid/J called NOD-SCID mice were used. Founders were obtained from Jackson Laboratories (Bar, Harbor, Maine, United States) and the colony was housed at the animal facility of University of Cantabria, Santander, Spain under aseptic conditions and veterinary control.
For the ONJ models, 8 week-old mice were anesthetised and the maxillary right first molar was extracted, as published using immunocompetent mice (16). Mice were treated with several intraperitoneal (IP) or intravenous (IV) (retro-orbital plexus) doses of 540 μg/kg of zoledronic acid. The first dose was always given 1 week before the dental extraction, with subsequent doses at weekly intervals. Some mice additionally received subcutaneous dexamethasone (10 mg/kg, 3 times a week). Mice were euthanized 1 week after the last zoledronic acid dose. The maxillary bones were dissected, fixed in formaldehyde and preserved in ethanol prior to study by micro-CT (Bruker). In some experimental mice, as well as in control mice of the same age which did not undergo any experimental procedures, the femur and the tibia were dissected and the trabecular bone volume was determined as previously described (17). After micro-CT analysis, bones were decalcified, paraffin-embedded, and stained with hematoxylin-eosin. Regions of denuded mucosa and bone necrosis (defined as 5 adjacent empty osteocytic lacunae [18]) were evaluated by 2 independent observers.
For the osteoporosis model, female 8-week old NOD-SCID mice were used. Under general anesthesia, both ovaries were exposed and removed. Mice receive opioid analgesia for pain management right after the intervention. A sham-operated group was also included. Eight weeks later, the animals were euthanized, and both the femora and the tibiae were removed and analyzed by micro-CT. T-test was assessed as statistical analysis to compare the differences between mice groups (p-values indicated).
Protocol approval was obtained from University of Cantabria Research Ethics Committee and Consejeria de Sanidad de Cantabria, as established by current regulations (code 2016-27930).
RESULTS
OSTEONECROSIS OF THE JAW MODEL
In this study, 27 mice were used to establish an osteonecrosis of the jaw (ONJ) model, receiving varying doses of zoledronic acid administered via IV or IP acces. Table I illustrates the different treatment regimens, including the number of doses, administration route, duration of the study following molar extraction, and the incidence of osteonecrosis observed among the mice.
Table I. Drug treatment and responses in the ONJ group.
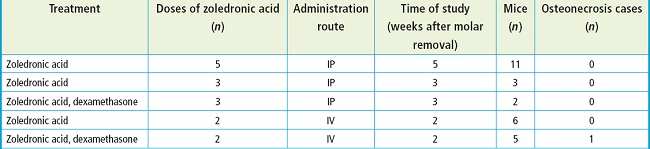
IP: intraperitoneal administration group; IV: intravenous administration group.
Micro-CT analysis confirmed the absence of the first molar, without abnormalities in the maxillary bone (Figs. 1 A and B). Only 1 out of the 27 mice studied showed some evidence of mild ONJ, with a small area of bone with several empty osteocytic lacunae. This mouse had been treated with 2 IV injections of zoledronic acid and dexamethasone (Figs. 1 C and D).
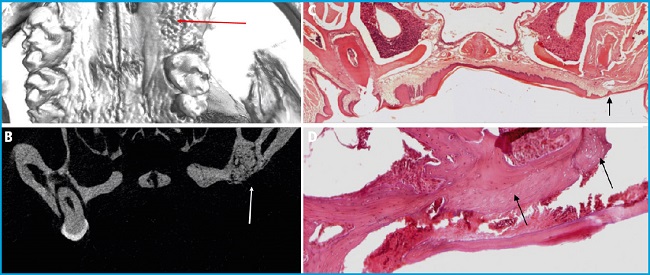
Figure 1. A. 3D reconstruction of the micro-CT analysis showing absence of first molar and cavity filled with new bone (red arrow). B. Coronal image, micro-CT analysis showing the absence of the first molar and the cavity filled with new bone (white arrow). C. Normal healing after tooth removal. HE staining showing complete regeneration of oral mucosa and filling of the cavity (black arrow). Image captured at 4× magnification. D. Mild bone necrosis. HE staining of the only mice showing an area of bone necrosis adjacent to the removed molar cavity (black arrows). Image captured at 10× magnification.
In some representative mice, we measured bone mass to confirm the bioactivity of zoledronic acid. As expected, trabecular bone volume was increased by zoledronic acid via IV or IP access (from a mean 30 ± 8 % to 48 ± 10 % at the femur, and 32 ± 6 % to 48 ± 12 % at the tibia).
OSTEOPOROSIS MODEL
In the evaluation of an osteoporosis model, 24 ovariectomized (OVX) mice and 11 controls (COVX) were successfully included for micro-CT analyses. Unexpectedly, bone mineral density (BMD), bone volume versus tissue volume (BV/TV) parameter, and cortical thickness were similar between the 2 groups (Fig. 2). Specifically, the mean BMD for the femur was 0.20 g/cm² in the OVX group, identical to the COVX group. The BV/TV mean values were 20.3 ± 4.7 % for OVX and 21.8 ± 3.2 for COVX. Hence, no significant differences were found in the bone measured parameters (BMD; p = 0.932; BV/TV p = 0.1507). Dissimilarities were not found either in the femur cortical thickness (p = 0.4242).
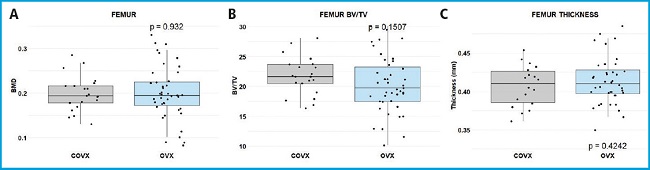
Figure 2. Comparative BMD and cortical thickness analyses with micro-CT. A. Femoral BMD of control (COVX, gray) and ovariectomized (OVX, blue) groups. B. Femoral BV/TV of COVX (gray) vs OVX (blue). C. Femoral cortical thickness of COVX (gray) and OVX (blue) groups. Statistical significance assessed via t-test (p-values indicated). Both lower limbs are represented for each mice as an individual point.
DISCUSSION
BPs are the most widely used antiresorptives in the treatment of OP and certain tumors. ONJ is a rare but serious side effect that can arise in patients on antiresorptives. As in other many complex disorders, ONJ seems to be the result of a combination of genetic and environmental factors. There are several well-established acquired risk factors associated with ONJ, such as previous dental extraction, periodontal disease, and the administration of BP (with differences depending on the type and administration route). Local infections and the immune response are also considered part of the ONJ pathogenesis. Different studies have proposed a potential role of Tγδ lymphocytes (18), neutrophils, and macrophages, as well as interleukins and proteins produced by cells involved in the immune system (19).
Despite molar extraction and the injection of high doses of zoledronic acid, only 1 out of the 27 studied mice developed histological findings consistent with mild ONJ. These results widely differ from the 45%-50% of ONJ cases described in studies in immunocompetent mice using a similar methodology (20,21).
Unlike immunocompetent mice, it seems that immunosuppressed mice tend not to develop ONJ even after an insult to the oral mucosa and having received high doses of BP and steroids. Although the mechanisms involved await further studies, we can speculate that it could be due to the immune deficiency per se or the sterile conditions in which those mice were maintained, which likely modified oral microbiota. These failed experiments reinforce the concept that factors apart from medication, such as the immune system and the microbiota, play an important role in the occurrence of ONJ after BP therapy.
In line with the ONJ model, the absence of a functional immune system in NOD.CB17-Prkdcscid/J mice, and/or the maintenance of the mice under sterile conditions seemed to prevent them from developing the expected bone loss after OVX. Studies using immunocompetent mice typically report a significant decrease in BMD and changes to bone architecture after 3 weeks following ovariectomy. For example, Smith et al. (2020) observed a significant reduction in BMD and BV/TV and significant increases in trabecular separation in C57BL/6 mice 8 weeks after ovariectomy, highlighting the accelerated bone loss in this model due to normal immune function (22). These differences underscore the critical role of the immune system in post-ovariectomy bone remodeling and the potential for unique disease progression pathways in immunocompromised conditions.
The immune system plays a pivotal role in bone homeostasis, with T cells, B cells, and cytokines influencing both bone resorption and formation. In particular, T cells have been involved in osteoclastogenesis through the production of RANKL, a key osteoclast-activating factor (23). Additionally, classic studies from the Pacifici and Manolagas groups confirmed the role played by cytokines, such as IL-1, IL-6, and tumor necrosis factor, produced by immune and other cells, in OVX-induced osteoporosis (24-26). The lack of an adaptive immune response in these immunodeficient mice could therefore significantly ameliorate the osteoclast-mediated bone resorption expected to occur after ovariectomy.
Moreover, gut microbiota has been increasingly recognized for its influence on bone density and health (27). Microbiota dysbiosis has been associated with inflammatory responses capable of promoting osteoclast activity, eventually leading to bone loss (28-30). The sterile conditions under which NOD.CB17-Prkdcscid/J mice are kept could impact their microbiota, potentially affecting the incidence and progression of bone loss. This concept would be consistent with reports showing that estrogen deficiency causes a gut microbiome-dependent expansion of Th17 cells and TNF-α-producing T cells. This may be of pathophysiological importance, because the blockade of Th17 cell and TNF+ T cell egress from the gut prevented OVX-induced bone loss (31).
In conclusion, the blunted ability to induce BP-related ONJ and OVX-related decrease in bone mass is a limitation for using immunodeficient mice as experimental models in osteoporosis research. On the other hand, they highlight the role of osteoinmmunological mechanisms, and perhaps body microbiota, in bone pathophysiology. The correlation between these mechanisms and their collective impact on bone health requires a broader perspective when investigating osteoporosis and should be taken into account when conducting studies with animal models.














