Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Archivos Españoles de Urología (Ed. impresa)
versión impresa ISSN 0004-0614
Arch. Esp. Urol. vol.60 no.4 may. 2007
History, evolution and application of robotic surgery in urology
Javier Romero Otero, Philippe Paparel, Dash Atreya, Karim Touijer and Bertrand Guillonneau.
Department of Urology. Memorial Sloan Kettering Cancer Center. New York City (NY). USA.
SUMMARY
Robotic is an antique concep. The first robots used in surgery were precise path systems in the 80´s. Stereotactic neurosurgery was the first field applying this devices. Based on these more complex devices were built: AESOP and Endoassist help the surgeon during the surgery. The surgical assistant will not fatigue and there will be no tremor of the camera. Finally the master-slave devices were developed. They are the most commenly used all around the world. They are involved many types surgery in. To evaluate the cost-effectiviness of robotics in surgery is our responsability. Robotics provides many advantages but also has a few disadvantages including expense.
Key words: Robotics. Robotic surgery. Robotic surgery history. Urologic history. Robotics and urology.
Introduction
Robotics concept is an attractive idea coming from far ago. The idea of artificial people dates at least from the ancient legend of Cadmus and the myth of Pygmalion. In ancient Greek, Ctesibius and Philon developed mechanical figures, known as automata, which were made for the pleasure and amusement of the wealthy.
The modern concept of robotics is derived from the Industrial Revolution. The term robot in Czech comes from forced labor. Karel Capek inspired by the unemployment generated by the introduction of mechanization during the Industrial Revolution, coined the term in his play Rossum´s Universal Robots (1921). The science fiction writer Isaac Asimov popularized the term in his books: Runaround (1942) and I, Robot (1950) (1).
Robotics in surgery:
In the late 1980s researchers at the National Aeronautics and Space Administration (NASA) Ames Research Centerand and engineers from the Stanford Research Institute (SRI) became interested in virtual reality and robotic technologies. Their joint efforts culminated in the development of a telepresence surgical system to improve dexterity in microscopic hand surgery. To demonstrate the efficiency of the system, 10 end-to-end femoral artery anastomoses in rats were performed. Complete and intact vascular flow was achieved (2). The original idea soon evolved from microscopic to macroscopic surgery. The definitive event was the development of surgical laparoscopy in Europe, which was later introduced in the USA by Perisat, a surgeon from the University Hospital of Bordeaux, France, who performed a laparoscopic cholecystectomy demonstration at the Society of American Gastrointestinal Surgeons meeting in Atlanta in 1989 (3). Laparoscopic surgery was ideal for the development of the new technology.
The US Department of Defense based upon the tele-surgery idea developed a new device: SRI Green telepresence Surgery System. It was a mobile, armored, operating room vehicle equipped with robotic surgical manipulators controlled remotely by a surgeon at a rear area mobile surgical hospital unit. An initial injured soldier could receive surgical assistance to control all the mortal lesions, mainly the vascular traumas, later he could receive the definitive surgery and care in a military hospital. Early studies demonstrated the systems feasibility in performing remote open trauma surgery, although operative times were 3 times that of the open techniques. Although the telepresence technologies have been never used in combat care, modern robotic surgery evolved from this seminal work (4).
In Europe there has been an effort to apply robotics to surgery. Schurr et al in Germany developed the first system equipped with arms enabled to move with six degrees of freedom and a three dimensional visualization system, called Advanced Robotic Telemanipulator for Minimally Invasive Surgery (ARTEMIS). Although and the ARTEMIS system was technically sophisticated, it has not been successfully comercialized (5).
Robotics has been applied to other fields of medicine including Traumatology, Neurosurgery and others. The application in these fields may have useful correlates in urologic surgery that should be considered. Between these the Programamable Universal Machine for Assembly (PUMA) 560 used by Kwoh et al for neuro-stereoctatic surgery (6), which was the first robot-assisted surgical procedure reported in the literature. Based on it Wickham et al began a robotic-surgery research program at the Imperial College of London. Secondary to this effort the Probot for prostatectomies (7) was built up. At the same time and following the initial idea Paul et al developed the ROBODOC (Integrated surgical systems, Sacramento, CA) for orthopedic surgery (8).
Later the AESOP was successfully applied to hold the endoscope during laparoscopy. Soon it was routinely used for the laparoscopic procedures in many reference centers (9).
Most of the robotic procedures are now made with the da Vinci system. As we mentioned before, laparoscopy was the key for the development of this new technology. Robotic surgery was developed based on the laparoscopic principles and experience, probably because robotic is just a type of laparoscopic procedure. Again laparoscopic surgeons were the pioneers doing the initial robotic procedures and their initial effort allowed the fast development of the new technology. We should mention that the first robotic radical prostatectomy was performed by Binder et al, in Germany, later Abbou et al, in France, reported the first one in the literature (10). Guillonneau et al reported the first robotic nephrectomy (11) and also the first lymphadenectomy for a prostate cancer (12).
Robotic systems classification:
One can disctinguish three different classes of robotics systems, from the more automated to the simpler (4):
1. Precise path systems, are pre-programmed mechanic devices to perform systematically repetitive and predefined movements. It requires no direct guidance from the surgeon. This class of device includes: the Surgeon Robot for Prostatectomies, designed to perform prostatic transurethral resections in a mechanical fashion (13); and the PAKY device to puncture the renal calix during the percutaneous kidney procedures (14).
2. Intern replacement surgical robots. Are an intermediate class between the previous group and the master-slave devices. They substitute the surgical assistant to perform tasks that require dexterity without tiring. This group includes the: Automated Endoscopic System for Optimal Positioning (AESOP) and Endoassist. Such devices function as endoscopic holders that can be directed by commands from the surgeon.
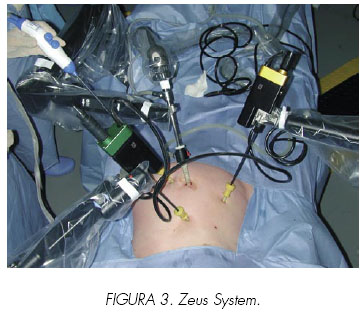
3. The master-slave device is the paradigm of robotics as it is referred today, even if it is the least automatic of all systems. It is composed of a computer console for surgeon interaction, from where he can remotely control the robotic tower that supports three or four robotic arms. The robotic device never moves independently without guidance from the surgeon. The arms precisely mimic the surgeon´s movements at the console within the patients body. Devices that meet these characteristics include: the da VinciTM Surgical System (Intuitive Surgical, Sunnyvale, California) and the Zeus Robotic Surgical System (Computer Motion, Goleta, California). These robotic system have computer controls with the aim to improve the surgeon´s dexterity and reduce tremor (15).
Currently available Robotic systems:
Five robotics systems have been approved by the Food and Drug Administration (FDA) to date. These devices are totally integrated and working in many centers: Neuromate, AESOP, Endoassist, da Vinci Surgical System and Zeus Surgical System. Since the merger of Intuitive Surgical and Computer Motion in June 2003, the Zeus Surgical System is no longer commercially available, but maintenance and replacement part support are still available, allowing several institutions to continue to use it.
1. AESOP: It consists of a single articulated arm with 4 degrees of freedom that holds the endoscope during laparoscopic procedures. In the first generation mode the movements were controlled by the surgeon with a foot-pedal, but soon after a voice-control device was developed. Its advantage over a surgical assistant is that it does not fatigue and holds the camera with stability. It has proven to be very efficient by allowing the surgeon to operate without an assistant dedicated to holding the camera, and eventually alone without increasing the operative time (16). Beside urology, it is used in many other surgical fields such as gynecology (17) and gastrointestinal surgery (18).
2. Endoassist: The Endoassist is very similar to the AESOP. It holds and positions the endoscope. The Endoassist may be controlled by means of a joystick or a headband sensor. The headband device emits infrared signals allowing the robot to track the surgeon´s head movement. When a foot switch is pressed, the robot moves the camera in the direction that the surgeon is looking on the monitor. Although it is as good as the AESOP it is not commonly used and little is published about it (19).
3. Neuromate: Is an image-guided, computer-controlled robotic system designed for stereotactic brain surgeries (20).
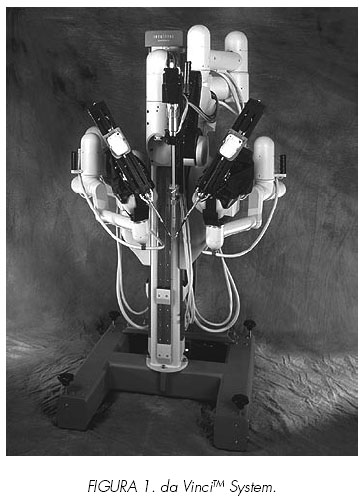
4. da VinciTM Surgical System: It is composed of 3 distinct parts: 1. The master console: The surgeon sits in an ergonomically designed chair to control the robot from the main computer (Figures 1 and 2). 2. An electronic tower holds the video, the light, a monitor for the assist and the insufflator. 3. The robotics tower or slave robot is positioned over the patient and holds three or four arms (Figure 3). During the surgery, both master and slave, must be in the same room.
In July 2000 it was approved by the FDA. The surgeon sits at the master console and controls the surgical field through a binocular port that displays true three dimensional viewing. This major visual advancement is in fact indispensable in master-slave machines since the surgeon has no sense about the position of the robotic arms and instruments within the space. The absence of proprioceptive sensation renders the understanding of the relationship of the instruments in the space dependent only upon the vision: therefore the three dimensional vision is a pre-requisite for any machines developed on the concept of a master-slave interaction. Two ergonomic manual controls allow the surgeon to make all the anatomical movements and then transmitted to the robotic arms at the patient. One of its major advantages is that when the surgeon looks through the binocular port, the computer integrates the surgical instrumentation (grasper, forceps, scissors...) in the patient as if they are the surgeons hands, so all the movements become intuitive (in the surgeon´s brain the instruments are exactly his hands). Foot pedals allow the control of the cautery. The robotic tower is placed at the patient side and can hold three or four arms. The central arm is to hold the endoscope and the others are articulated arms with seven degrees of freedom that mimic the surgeons movements at the master console.
Several advantages and disadvantages have been related with this device. Advantages are: 3D vision allows more precise movements, articulated arms with endo-wrist that provide the possibility of seven degrees of freedom, information computer processing eliminates the surgeon tremor and the possibility for the surgeon to operate while sitting and a ten times image magnification.
The main disadvantage is its absolute lack of sensitivity making it impossible to interpret force or any tactile feed-back. Receive it is expensive creates space problems making assisting when working in a small anatomical space the arms inacurate. When may interfere one to each other.
5. Zeus Surgical System: A master console allows the surgeon to control three independent articulated arms positioned at the patient. One of them is an AESOP to hold the endoscope. The two other arms are similar and based on the same technology. This device provides six degrees of freedom. Although initially it was developed with a two dimensional viewing system, the final version was provided with a 3-D system (Figures 3 and 4). In 2001, Marescaux from Strasboug performed the first and so far unique transatlantic surgery in history. He performed from New York, USA, an uneventful cholecystectomy on a patient in Strasbourg, France (21).
General applications of robotic surgery today: The first robotic surgical series was reported by Cadiere et al (22) in Europe from March 1997, 146 cases were reported. Most of them were gastrointestinal procedures, but radical prostatectomy and varicocelectomy were also performed. Across the Atlantic, in the EEUU, Talami et al reported his initial robotic experience in using the da VinciTM system, from June 2000 to June 2001 (23), 211 cases. The majority of them were gastrointestinal procedures but a living donor nephrectomy and an adrenalectomy were performed.
Since then all surgical specialties have been attracted to robotic surgery. Robotic surgery has demonstrated its ability to perform most the surgical techniques. In cardio-thoracic surgery the following procedures have been reported: mitral (24) and aortic (25) valve surgery, coronary (26) and internal mammary (27) artery bypass, atrial septal defect repair (28), implantation of defibrillator (29), resection of an esophageal cyst (30), resection of mediastinal parathyroids (31) and bronchoplasties (32). The vascular surgeons have performed their most important procedures: aorto-iliac bypass and aortic aneurysm repair (33).
The patients size is not a contraindication for robotic surgery and many techniques have been done in pediatric patients: pyeloplasties (34), heminephrectomies (35) and appendicovesicostomy (Mitrofanoff procedure) (36).
At present, the major application of robotic surgery has been in abdominal surgery, including digestive, gynecologic and urologic surgery. Pancreatic resections (37), cholecystectomies (38), Roux-en-Y gastric bypass and colectomies (39) have been described. In the gynecologic area the robot has been used.
For uterine horn anastomosis (40), tubal reanastomosis (41,42), endometriosis surgery, myomectomies, hysterectomies (43), sacrocolpopexy for vaginal vault prolapse (44) and vesicovaginal fistulas repair (45).
Robotics in urology is not the future, but is the present for several operations and is routinely offered in many institutions these procedures include: pelvic floor reconstructions (46), vaso-vasostomies (47), cystectomies and intracorporeal neobladder (48), nephrectomies and adrenalectomies (49), pyeloplasties (50), living donor nephrectomy for kidney trasplantation (51), partial nephrectomies (52) and of course radical prostatectomy that is the most common surgery using this technology at the present time.
Other robotic systems involved in Urology:
The master-slave is the most important robotic systems, but not the only one used for urologic surgery. Many other robots have been employed during urologic surgery:
1. Surgeon Robot for Prostatectomies (Probot) (53): Represents the first application of robotics technology in urology. It was developed at Guy´s Hospital and Imperial College of London.
It is a transurethral resector controlled by a precise paths device. Before surgery, thanks to transrectal ultrasonography, the prostate size was measured and then the system was programed to perform repetitive movements to resect the tissue, always respecting the prostate limits. During the procedure the surgeon could watch the surgery in a monitor (as we usually do). At the end of the operation the surgeon inspected and ensured hemastasis at the prostate surface. Although initially they reported very good results, they did not report long-term nor commercialize the system.
2. Robotic System for Percutaneous Access (PAKY) (14): developed by Johns Hopkins University, basically is a robotic arm with seven degrees of freedom and a needle at the end. It was helpful to achieve renal percutaneous acces.
Conclusions
Robotic is an old concept. The joint efforts of many have contributed to the success we enjoy today. Each new device is an improvement the previous one, but always based previous experience and knowledge. Finally the master-slave device has emerged as the most useful and is being applied in most of the surgeries. Other types of robots such AESOP are also helpful in daily laparoscopic practice.
Robots are supposedly designed to help surgeons achieve better results for patients and to make complex procedures easier. It is the obligation of academic medicine to measure the efficacy and efficency of these machines.
![]() Correspondence:
Correspondence:
Javier Romero Otero, M.D.
Department of Urology
Memorial Sloan Kettering Cancer Center
353 E 68th st,
ZIP: 10028. New York City (NY).
romerooj@mskcc.org
References and recommended readings (*of special interest, **of outstanding interest)
**1. EWING, D. R.; PIGAZZI, A.; WANG, Y. y cols.: Robots in the operating room--the history. Semin Laparosc Surg, 11: 63, 2004. [ Links ]
2. HILL, J. W.; HOLST, P. A.; JENSEN, J. F. y cols.: Telepresence interface with applications to microsurgery and surgical simulation. Stud Health Technol Inform, 50: 96, 1998. [ Links ]
3. PERISSAT, J.; COLLET, D. R.; BELLIARD, R.: Gallstones: laparoscopic treatment, intracorporeal lithotripsy followed by cholecystostomy or cholecystectomy--a personal technique. Endoscopy, 21 Suppl 1: 373, 1989. [ Links ]
**4. NGUYEN, M. M.; DAS, S.: The evolution of robotic urologic surgery. Urol Clin North Am, 31: 653, 2004. [ Links ]
5. SCHURR, M. O.; BUESS, G.; NEISIUS, B. y cols.: Robotics and telemanipulation technologies for endoscopic surgery. A review of the ARTEMIS project. Advanced Robotic Telemanipulator for Minimally Invasive Surgery. Surg Endosc, 14: 375, 2000. [ Links ]
6. KWOH, Y. S.; HOU, J.; JONCKHEERE, E. A. y cols.: A robot with improved absolute positioning accuracy for CT guided stereotactic brain surgery. IEEE Trans Biomed Eng, 35: 153, 1988. [ Links ]
7. WICKHAM, J.: Minimally invasive therapy. Health Trends, 23: 6, 1991. [ Links ]
8. PAUL, H. A.; BARGAR, W. L.; MITTLESTADT, B. y cols.: Development of a surgical robot for cementless total hip arthroplasty. Clin Orthop Relat Res: 57, 1992. [ Links ]
9. GUILLONNEAU, B.; VALLANCIEN, G.: Laparoscopic radical prostatectomy: the Montsouris technique. J Urol, 163: 1643, 2000. [ Links ]
10. ABBOU, C. C.; HOZNEK, A.; SALOMON, L. y cols.: Laparoscopic radical prostatectomy with a remote controlled robot. J Urol, 165: 1964, 2001. [ Links ]
11. GUILLONNEAU, B.; JAYET, C.; TEWARI, A. y cols.: Robot assisted laparoscopic nephrectomy. J Urol, 166: 200, 2001. [ Links ]
12. GUILLONNEAU, B.; CAPPELE, O.; MARTINEZ, J. B. y cols.: Robotic assisted, laparoscopic pelvic lymph node dissection in humans. J Urol, 165: 1078, 2001. [ Links ]
*13. DAVIES, B. L.; HIBBERD, R. D.; NG, W. S. y cols.: The development of a surgeon robot for prostatectomies. Proc Inst Mech Eng [H], 205: 35, 1991. [ Links ]
14. CADEDDU, J. A.; BZOSTEK, A.; SCHREINER, S. y cols: A robotic system for percutaneous renal access. J Urol, 158: 1589, 1997. [ Links ]
*15. GUILLONNEAU, B.: What robotics in urology? A current point of view. Eur Urol, 43: 103, 2003. [ Links ]
16. KAVOUSSI, L. R.; MOORE, R. G.; ADAMS, J. B. y cols.: Comparison of robotic versus human laparoscopic camera control. J Urol, 154: 2134, 1995. [ Links ]v17. METTLER, L.; IBRAHIM, M.; JONAT, W.: One year of experience working with the aid of a robotic assistant (the voice-controlled optic holder AESOP) in gynaecological endoscopic surgery. Hum Reprod, 13: 2748, 1998. [ Links ]
18. GEIS, W. P.; KIM, H. C.; BRENNAN, E. J., JR. y cols.: Robotic arm enhancement to accommodate improved efficiency and decreased resource utilization in complex minimally invasive surgical procedures. Stud Health Technol Inform, 29: 471, 1996. [ Links ]
19. AIONO, S.; GILBERT, J. M.; SOIN, B. y cols.: Controlled trial of the introduction of a robotic camera assistant (EndoAssist) for laparoscopic cholecystectomy. Surg Endosc, 16: 1267, 2002. [ Links ]
20. LI, Q. H.; ZAMORANO, L.; PANDYA, A. y cols.: The application accuracy of the NeuroMate robot--A quantitative comparison with frameless and frame-based surgical localization systems. Comput Aided Surg, 7: 90, 2002. [ Links ]
21. MARESCAUX, J.; LEROY, J.; GAGNER, M. y cols.: Transatlantic robot-assisted telesurgery. Nature, 413: 379, 2001. [ Links ]
*22. CADIERE, G. B.; HIMPENS, J.; GERMAY, O. y cols.: Feasibility of robotic laparoscopic surgery: 146 cases. World J Surg, 25: 1467, 2001. [ Links ]
23. TALAMINI, M. A.; CHAPMAN, S.; HORGAN, S. y cols.: A prospective analysis of 211 robotic-assisted surgical procedures. Surg Endosc, 17: 1521, 2003. [ Links ]
24. MURPHY, D. A.; MILLER, J. S.; LANGFORD, D. A. y cols.: Endoscopic robotic mitral valve surgery. J Thorac Cardiovasc Surg, 132: 776, 2006. [ Links ]
25. FOLLIGUET, T. A.; VANHUYSE, F.; KONSTANTINOS, Z. y cols.: Early experience with robotic aortic valve replacement. Eur J Cardiothorac Surg, 28: 172, 2005. [ Links ]
26. TURNER, W. F., JR., SLOAN, J. H.: Robotic-assisted coronary artery bypass on a beating heart: initial experience and implications for the future. Ann Thorac Surg, 82: 790, 2006. [ Links ]
27. BOYD, B.; UMANSKY, J.; SAMSON, M. y cols.: Robotic harvest of internal mammary vessels in breast reconstruction. J Reconstr Microsurg, 22: 261, 2006. [ Links ]
28. BONAROS, N.; SCHACHNER, T.; OEHLINGER, A. y cols.: Robotically assisted totally endoscopic atrial septal defect repair: insights from operative times, learning curves, and clinical outcome. Ann Thorac Surg, 82: 687, 2006. [ Links ]
29. SHALABY, A.; SHARMA, M. S.; ZENATI, M. A.: Robotic implantation of a multichamber cardiac resynchronization therapy defibrillator. Pacing Clin Electrophysiol, 29: 906, 2006. [ Links ]
30. FERNANDO, H. C.; ERDEM, C. C.; DALY, B. y cols.: Robotic assisted thoracic surgery for resection of an esophageal cyst. Dis Esophagus, 19: 509, 2006. [ Links ]
31. BODNER,; PROMMEGGER,; PROFANTER. y cols.: Thoracoscopic resection of mediastinal parathyroids: current status and future perspectives. Minim Invasive Ther Allied Technol, 13: 199, 2004. [ Links ]
32. ISHIKAWA, N.; SUN, Y. S.; NIFONG, L. W. y cols.: Thoracoscopic robot-assisted bronchoplasty. Surg Endosc, 20: 1782, 2006. [ Links ]
33. STADLER, P.; MATOUS, P.; VITASEK, P. y cols.: Robot-assisted aortoiliac reconstruction: A review of 30 cases. J Vasc Surg, 44: 915, 2006. [ Links ]
34. ATUG, F.; WOODS, M.; BURGESS, S. V. y cols.: Robotic assisted laparoscopic pyeloplasty in children. J Urol, 174: 1440, 2005. [ Links ]
35. PEDRAZA, R.; PALMER, L.; MOSS, V. y cols.: Bilateral robotic assisted laparoscopic heminephroureterectomy. J Urol, 171: 2394, 2004. [ Links ]
36. PEDRAZA, R.; WEISER, A.; FRANCO, I.: Laparoscopic appendicovesicostomy (Mitrofanoff procedure) in a child using the da Vinci robotic system. J Urol, 171: 1652, 2004. [ Links ]
37. HSU, S. D.; WU, H. S.; KUO, C. L. y cols.: Robotic-assisted laparoscopic resection of ectopic pancreas in the posterior wall of gastric high body: case report and review of the literature. World J Gastroenterol, 11: 7694, 2005. [ Links ]
38. TANOUE, K.; YASUNAGA, T.; KOBAYASHI, E. y cols.: Laparoscopic cholecystectomy using a newly developed laparoscope manipulator for 10 patients with cholelithiasis. Surg Endosc, 20: 753, 2006. [ Links ]
39. ALI, M. R.; BHASKERRAO, B.; WOLFE, B. M.: Robot-assisted laparoscopic Roux-en-Y gastric bypass. Surg Endosc, 19: 468, 2005. [ Links ]
40. MARGOSSIAN, H.; GARCIA-RUIZ, A.; FALCONE, T. y cols.: Robotically assisted laparoscopic microsurgical uterine horn anastomosis. Fertil Steril, 70: 530, 1998. [ Links ]
41. FALCONE, T.; GOLDBERG, J.; GARCIA-RUIZ, A. y cols.: Full robotic assistance for laparoscopic tubal anastomosis: a case report. J Laparoendosc Adv Surg Tech A, 9: 107, 1999. [ Links ]
42. DEGUELDRE, M.; VANDROMME, J.; HUONG, P. T. y cols.: Robotically assisted laparoscopic microsurgical tubal reanastomosis: a feasibility study. Fertil Steril, 74: 1020, 2000. [ Links ]
43. FIORENTINO, R. P.; ZEPEDA, M. A.; GOLDSTEIN, B. H. y cols.: Pilot study assessing robotic laparoscopic hysterectomy and patient outcomes. J Minim Invasive Gynecol, 13: 60, 2006. [ Links ]
44. ELLIOTT, D. S.; KRAMBECK, A. E.; CHOW, G. K.: Long-term results of robotic assisted laparoscopic sacrocolpopexy for the treatment of high grade vaginal vault prolapse. J Urol, 176: 655, 2006. [ Links ]
45. SUNDARAM, B. M.; KALIDASAN, G.; HEMAL, A. K.: Robotic repair of vesicovaginal fistula: case series of five patients. Urology, 67: 970, 2006. [ Links ]
*46. BEECKEN, W. D.; WOLFRAM, M.; ENGL, T. y cols.: Robotic-assisted laparoscopic radical cystectomy and intra-abdominal formation of an orthotopic ileal neobladder. Eur Urol, 44: 337, 2003. [ Links ]
47. FLEISCHMANN, N. B.; NITTI, V. W.: Pelvic floor reconstruction: state-of-the-art and beyond. Urol Clin North Am, 31: 757, 2004. [ Links ]
48. SCHIFF, J.; LI, P. S.; GOLDSTEIN, M.: Robotic microsurgical vasovasostomy and vasoepididymostomy: a prospective randomized study in a rat model. J Urol, 171: 1720, 2004. [ Links ]
49. GILL, I. S.; SUNG, G. T.; HSU, T. H. y cols.: Robotic remote laparoscopic nephrectomy and adrenalectomy: the initial experience. J Urol, 164: 2082, 2000. [ Links ]
50. SUNG, G. T.; GILL, I. S.; HSU, T. H.: Robotic-assisted laparoscopic pyeloplasty: a pilot study. Urology, 53: 1099, 1999. [ Links ]
51. HORGAN, S.; VANUNO, D.; SILERI, P. y cols.: Robotic-assisted laparoscopic donor nephrectomy for kidney transplantation. Transplantation, 73: 1474, 2002. [ Links ]
52. PHILLIPS, C. K.; TANEJA, S. S.; STIFELMAN, M. D.: Robot-assisted laparoscopic partial nephrectomy: the NYU technique. J Endourol, 19: 441, 2005. [ Links ]
53. HARRIS, S. J.; ARAMBULA-COSIO, F.; MEI, Q. y cols.: The Probot--an active robot for prostate resection. Proc Inst Mech Eng [H], 211: 317, 1997. [ Links ]











 texto en
texto en